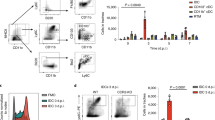
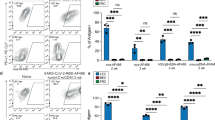
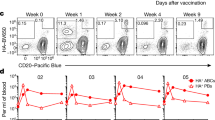
Abstract
A major pathway for B cell acquisition of lymph-borne particulate antigens relies on antigen capture by subcapsular sinus macrophages of the lymph node. Here we tested whether this mechanism is also important for humoral immunity to inactivated influenza virus. By multiple approaches, including multiphoton intravital imaging, we found that antigen capture by sinus-lining macrophages was important for limiting the systemic spread of virus but not for the generation of influenza-specific humoral immunity. Instead, we found that dendritic cells residing in the lymph node medulla use the lectin receptor SIGN-R1 to capture lymph-borne influenza virus and promote humoral immunity. Thus, our results have important implications for the generation of durable humoral immunity to viral pathogens through vaccination.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Pulendran, B. & Ahmed, R. Translating innate immunity into immunological memory: implications for vaccine development. Cell 124, 849–863 (2006).
Gerhard, W., Mozdzanowska, K., Furchner, M., Washko, G. & Maiese, K. Role of the B-cell response in recovery of mice from primary influenza virus infection. Immunol. Rev. 159, 95–103 (1997).
Gretz, J.E., Anderson, A.O. & Shaw, S. Cords, channels, corridors and conduits: critical architectural elements facilitating cell interactions in the lymph node cortex. Immunol. Rev. 156, 11–24 (1997).
Kosco-Vilbois, M.H., Gray, D., Scheidegger, D. & Julius, M. Follicular dendritic cells help resting B cells to become effective antigen-presenting cells: Induction of B7/BB1 and upregulation of major histocompatibility complex class II molecules. J. Exp. Med. 178, 2055–2066 (1993).
Allen, C.D. & Cyster, J.G. Follicular dendritic cell networks of primary follicles and germinal centers: phenotype and function. Semin. Immunol. 20, 14–25 (2008).
MacLennan, I.C. Germinal centers. Annu. Rev. Immunol. 12, 117–139 (1994).
Junt, T. et al. Subcapsular sinus macrophages in lymph nodes clear lymph-borne viruses and present them to antiviral B cells. Nature 450, 110–114 (2007).
Phan, T.G., Grigorova, I., Okada, T. & Cyster, J.G. Subcapsular encounter and complement-dependent transport of immune complexes by lymph node B cells. Nat. Immunol. 8, 992–1000 (2007).
Qi, H., Egen, J.G., Huang, A.Y. & Germain, R.N. Extrafollicular activation of lymph node B cells by antigen-bearing dendritic cells. Science 312, 1672–1676 (2006).
Carrasco, Y.R. & Batista, F.D. B cells acquire particulate antigen in a macrophage-rich area at the boundary between the follicle and the subcapsular sinus of the lymph node. Immunity 27, 160–171 (2007).
Roozendaal, R. et al. Conduits mediate transport of low-molecular-weight antigen to lymph node follicles. Immunity 30, 264–276 (2009).
Martinez-Pomares, L. & Gordon, S. Antigen presentation the macrophage way. Cell 131, 641–643 (2007).
Bajenoff, M. & Germain, R.N. B cell follicle development remodels the conduit system and allows soluble antigen delivery to follicular dendritic cells. Blood 114, 4989–4997 (2009).
Pape, K.A., Catron, D.M., Itano, A.A. & Jenkins, M.K. The humoral immune response is initiated in lymph nodes by B cells that acquire soluble antigen directly in the follicles. Immunity 26, 491–502 (2007).
Gordon, S. & Taylor, P.R. Monocyte and macrophage heterogeneity. Nat. Rev. Immunol. 5, 953–964 (2005).
Phan, T.G., Green, J.A., Gray, E.E., Xu, Y. & Cyster, J.G. Immune complex relay by subcapsular sinus macrophages and noncognate B cells drives antibody affinity maturation. Nat. Immunol. 10, 786–793 (2009).
Taylor, P.R. et al. Macrophage receptors and immune recognition. Annu. Rev. Immunol. 23, 901–944 (2005).
Mebius, R.E. & Kraal, G. Structure and function of the spleen. Nat. Rev. Immunol. 5, 606–616 (2005).
Geijtenbeek, T.B. et al. Marginal zone macrophages express a murine homologue of DC-SIGN that captures blood-borne antigens in vivo. Blood 100, 2908–2916 (2002).
Kang, Y.S. et al. SIGN-R1, a novel C-type lectin expressed by marginal zone macrophages in spleen, mediates uptake of the polysaccharide dextran. Int. Immunol. 15, 177–186 (2003).
Taylor, P.R. et al. The role of SIGNR1 and the β-glucan receptor (dectin-1) in the nonopsonic recognition of yeast by specific macrophages. J. Immunol. 172, 1157–1162 (2004).
Kang, Y.S. et al. The C-type lectin SIGN-R1 mediates uptake of the capsular polysaccharide of Streptococcus pneumoniae in the marginal zone of mouse spleen. Proc. Natl. Acad. Sci. USA 101, 215–220 (2004).
Balazs, M., Martin, F., Zhou, T. & Kearney, J. Blood dendritic cells interact with splenic marginal zone B cells to initiate T-independent immune responses. Immunity 17, 341–352 (2002).
Berney, C. et al. A member of the dendritic cell family that enters B cell follicles and stimulates primary antibody responses identified by a mannose receptor fusion protein. J. Exp. Med. 190, 851–860 (1999).
Wykes, M., Pombo, A., Jenkins, C. & MacPherson, G.G. Dendritic cells interact directly with naive B lymphocytes to transfer antigen and initiate class switching in a primary T-dependent response. J. Immunol. 161, 1313–1319 (1998).
Kawai, T. et al. Anti-influenza A virus activities of mannan-binding lectins and bovine conglutinin. J. Vet. Med. Sci. 69, 221–224 (2007).
Shi, L. et al. Mannose-binding lectin-deficient mice are susceptible to infection with Staphylococcus aureus. J. Exp. Med. 199, 1379–1390 (2004).
Bedoui, S. et al. Cross-presentation of viral and self antigens by skin-derived CD103+ dendritic cells. Nat. Immunol. 10, 488–495 (2009).
Randolph, G.J., Inaba, K., Robbiani, D.F., Steinman, R.M. & Muller, W.A. Differentiation of phagocytic monocytes into lymph node dendritic cells in vivo. Immunity 11, 753–761 (1999).
Bot, A., Casares, S., Bot, S., von Boehmer, H. & Bona, C. Cellular mechanisms involved in protection against influenza virus infection in transgenic mice expressing a TCR receptor specific for class II hemagglutinin peptide in CD4+ and CD8+ T cells. J. Immunol. 160, 4500–4507 (1998).
Kang, Y.S. et al. A dominant complement fixation pathway for pneumococcal polysaccharides initiated by SIGN-R1 interacting with C1q. Cell 125, 47–58 (2006).
Lindquist, R.L. et al. Visualizing dendritic cell networks in vivo. Nat. Immunol. 5, 1243–1250 (2004).
Jung, S. et al. In vivo depletion of CD11c+ dendritic cells abrogates priming of CD8+ T cells by exogenous cell-associated antigens. Immunity 17, 211–220 (2002).
Probst, H.C. et al. Histological analysis of CD11c-DTR/GFP mice after in vivo depletion of dendritic cells. Clin. Exp. Immunol. 141, 398–404 (2005).
Sha, Z. & Compans, R.W. Induction of CD4+ T-cell-independent immunoglobulin responses by inactivated influenza virus. J. Virol. 74, 4999–5005 (2000).
Lee, B.O. et al. CD4 T cell-independent antibody response promotes resolution of primary influenza infection and helps to prevent reinfection. J. Immunol. 175, 5827–5838 (2005).
Taylor, P.R. et al. Development of a specific system for targeting protein to metallophilic macrophages. Proc. Natl. Acad. Sci. USA 101, 1963–1968 (2004).
Schwickert, T.A. et al. In vivo imaging of germinal centres reveals a dynamic open structure. Nature 446, 83–87 (2007).
Miller, M.J., Wei, S.H., Cahalan, M.D. & Parker, I. Autonomous T cell trafficking examined in vivo with intravital two-photon microscopy. Proc. Natl. Acad. Sci. USA 100, 2604–2609 (2003).
Neth, O., Jack, D.L., Johnson, M., Klein, N.J. & Turner, M.W. Enhancement of complement activation and opsonophagocytosis by complexes of mannose-binding lectin with mannose-binding lectin-associated serine protease after binding to Staphylococcus aureus. J. Immunol. 169, 4430–4436 (2002).
Shortman, K. & Naik, S.H. Steady-state and inflammatory dendritic-cell development. Nat. Rev. Immunol. 7, 19–30 (2007).
Itano, A.A. & Jenkins, M.K. Antigen presentation to naive CD4 T cells in the lymph node. Nat. Immunol. 4, 733–739 (2003).
Sixt, M. et al. The conduit system transports soluble antigens from the afferent lymph to resident dendritic cells in the T cell area of the lymph node. Immunity 22, 19–29 (2005).
Cinamon, G., Zachariah, M.A., Lam, O.M., Foss, F.W. Jr. & Cyster, J.G. Follicular shuttling of marginal zone B cells facilitates antigen transport. Nat. Immunol. 9, 54–62 (2008).
Ferguson, A.R., Youd, M.E. & Corley, R.B. Marginal zone B cells transport and deposit IgM-containing immune complexes onto follicular dendritic cells. Int. Immunol. 16, 1411–1422 (2004).
Guinamard, R., Okigaki, M., Schlessinger, J. & Ravetch, J.V. Absence of marginal zone B cells in Pyk-2-deficient mice defines their role in the humoral response. Nat. Immunol. 1, 31–36 (2000).
Pozdnyakova, O., Guttormsen, H.K., Lalani, F.N., Carroll, M.C. & Kasper, D.L. Impaired antibody response to group B streptococcal type III capsular polysaccharide in C3- and complement receptor 2-deficient mice. J. Immunol. 170, 84–90 (2003).
Sonoda, E. et al. B cell development under the condition of allelic inclusion. Immunity 6, 225–233 (1997).
Gack, M.U., S.Y., Joo, C.H., Urano, T., Liang, C., Sun, L., Takeuchi, O., Akira, S., Chen, Z., Inoue, S. & Jung, J.U. TRIM25 RING-finger E3 ubiquitin ligase is essential for RIG-I-mediated antiviral activity. Nature 446, 916–920 (2007).
Gonzalez, S.F., Jayasekera, J.P. & Carroll, M.C. Complement and natural antibody are required in the long term memory response to influenza virus. Vaccine 26, I86–I93 (2008).
Martinez-Pomares, L. et al. Fc chimeric protein containing the cysteine-rich domain of the murine mannose receptor binds to macrophages from splenic marginal zone and lymph node subcapsular sinus and to germinal centers. J. Exp. Med. 184, 1927–1937 (1996).
Woller, E.K. & Cloninger, M.J. The lectin-binding properties of six generations of mannose-functionalized dendrimers. Org. Lett. 4, 7–10 (2002).
Barrington, R.A., Borde, M., Rao, A. & Carroll, M.C. Involvement of NFAT1 in B cell self-tolerance. J. Immunol. 177, 1510–1515 (2006).
Miller, M.J., Wei, S.H., Parker, I. & Cahalan, M.D. Two-photon imaging of lymphocyte motility and antigen response in intact lymph node. Science 296, 1869–1873 (2002).
Acknowledgements
We thank J. Nolting (Dana Farber Cancer Institute) for hemagglutinin–T cell antigen receptor–transgenic mice; M. Nussenzweig (Rockefeller University) for CD11c-eYFP mice; R. Steinman (Rockefeller University) for the anti-SIGN-R1 hybridoma; K. Rajewsky (Harvard Medical School) for B1.8 mice; W. Gerhard (Wistar Institute) for the anti-hemagglutinin hybridoma; A. Garcia-Sastre (Mount Sinai School of Medicine) for influenza A/PR/8; J. Jensenius (Aarhus University) and S. Thiel (Aarhus University) for anti-MBL and technical advice; T. Mempel for the use of microscopy facilities and technical assistance; and H. Leung, E. Marino, M. Ericsson, H. Kim and A. Gillmore for technical assistance. Supported by the US National Institutes of Health (5 R01 AI039246, 1 P01 AI078897 and 5 R01 AI067706 to M.C.C., RO1 GM62444 to M.J.C. and RO1 DK074500 to S.J.T.) and the Seventh Framework Programme of the European Union (Marie Curie International Outgoing Fellowship 220044 to S.F.G.).
Author information
Authors and Affiliations
Contributions
S.J.T. and M.C.C. directed the study, designed experiments, analyzed and interpreted results and wrote the manuscript; S.F.G., V.L.-K., M.P.K., L.A.P., S.E.D. and Y.-A.K. designed experiments, analyzed and interpreted results; M.J.C. prepared dendrimer; and L.M.-P. and S.G. contributed reagents and helped to interpret results.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–7 and Methods (PDF 3443 kb)
Supplementary Movie 1
Subcapsular sinus macrophages (SSM) and medullary macrophages (MM) bind influenza virus in the draining LN. WT mice were pretreated with CD169 (green) and F4/80 (blue) antibodies to label SCS and MM respectively. Mice then prepared for MP-IVM, whole LN imaged and fluorescently labeled PR8 (red) was injected at time = 0 min. PR8 localization in the LN was imaged by MP-IVM at 15 s intervals for 30 min. PR8 (red) enters the SCS area within 8 min p.i. and remains in the SCS at 30 min where it colocalizes with CD169+ SSM and F4/80+ MM. Results are representative of 6 independent experiments, n = 6 mice total. (AVI 3197 kb)
Supplementary Movie 2
Mannose dendrimer blocks binding of PR8 to SSM, but not MM. Mice pretreated with CD169 (green) and anti-CD35 (blue) Ab to label SSM and FDC in vivo. Mice were subsequently treated with 40 μg of dendrimer in the footpad and were prepared for whole LN MP-IVM. Images were recorded beginning at time of fluorescently labeled PR8 injection (red, t = 0) at 15 sec intervals for 30 min. This movie demonstrates that following pretreatment with dendrimer, PR8 (red) does not bind to CD169+ SSM, but instead drains to the LN medulla where it binds to MM. Movie for 30 min p.i. Results are representative of 4 independent experiments, n = 4 mice total. (AVI 2922 kb)
Supplementary Movie 3
Medullary CD11c-EYFP cells bind virus is SIGN-R1 dependent. MP-IVM recordings of the LN medulla of a CD11c-YFP mouse (left images) and a CD11c-EYFP mouse treated with mAb against SIGN-R1 (22D1) to transiently knockout (TKO) SIGN-R1 expression (right images). The medulla of the popliteal LN were examined by MP-IVM beginning at t = 0 when PR8 (red) was injected into the footpad. Images were acquired every 30 sec for 120 min. Virus up take by CD11c-EYFP+ cells was substantially reduced in SIGN-R1 TKO mice, indicating that SIGN-R1 is crucial for CD11c-EYFP+ cells to bind virus. Results are representative of 4 independent experiments, n = 8 mice total. (MOV 12283 kb)
Supplementary Movie 4A
CD11c-EYFP cells bind PR8 and move to the FDC region. CD11c-EYFP mice were pretreated with anti-CD35 to label follicles and prepared for MP-IVM. An area of the LN was chosen for further analysis where the medullary region and FDC region occurred in one field of the MP (medullary region = top left, FDC region = bottom right). A baseline recording was performed for 60 min (a) and then fluorescently labeled PR8 (red) was injected into the footpad. MP-IVM recordings were continued for a further 60 min (b,c). 50 randomly chosen CD11c-EYFP+ cells were tracked prior to injection of PR8 (a) and then 50 randomly chosen CD11c-EYFP+ cells that captured PR8 (b) and 50 randomly chosen CD11c-EYFP+ cells which did not capture PR8 were also tracked (c). Results are representative of 4 independent experiments, n = 4 mice total. (AVI 2615 kb)
Supplementary Movie 4B
CD11c-EYFP cells bind PR8 and move to the FDC region. CD11c-EYFP mice were pretreated with anti-CD35 to label follicles and prepared for MP-IVM. An area of the LN was chosen for further analysis where the medullary region and FDC region occurred in one field of the MP (medullary region = top left, FDC region = bottom right). A baseline recording was performed for 60 min (a) and then fluorescently labeled PR8 (red) was injected into the footpad. MP-IVM recordings were continued for a further 60 min (b,c). 50 randomly chosen CD11c-EYFP+ cells were tracked prior to injection of PR8 (a) and then 50 randomly chosen CD11c-EYFP+ cells that captured PR8 (b) and 50 randomly chosen CD11c-EYFP+ cells which did not capture PR8 were also tracked (c). Results are representative of 4 independent experiments, n = 4 mice total. (AVI 3213 kb)
Supplementary Movie 4C
CD11c-EYFP cells bind PR8 and move to the FDC region. CD11c-EYFP mice were pretreated with anti-CD35 to label follicles and prepared for MP-IVM. An area of the LN was chosen for further analysis where the medullary region and FDC region occurred in one field of the MP (medullary region = top left, FDC region = bottom right). A baseline recording was performed for 60 min (a) and then fluorescently labeled PR8 (red) was injected into the footpad. MP-IVM recordings were continued for a further 60 min (b,c). 50 randomly chosen CD11c-EYFP+ cells were tracked prior to injection of PR8 (a) and then 50 randomly chosen CD11c-EYFP+ cells that captured PR8 (b) and 50 randomly chosen CD11c-EYFP+ cells which did not capture PR8 were also tracked (c). Results are representative of 4 independent experiments, n = 4 mice total. (AVI 3405 kb)
Supplementary Movie 5
Virus binding in the popliteal LN is MBL and SIGN-R1 dependent. MBL-deficient mice pretreated with SIGN-R1 were pretreated with MOMA-1 (green), F4/80 and 8C12 (blue) to label the SCS, medullary region and follicles, respectively. Virus localization in the LN was visualized using MP-IVM following injection of fluorescently labeled PR8 (red) into the footpad, time = 0 min, recordings made every 15 s for 60 min. Negligible virus is observed in the SCS or in the medullary region in MBL deficient mice pretreated with SIGN-R1 blocking antibodies. Results are representative of 3 independent experiments, n = 3 mice total. (AVI 5016 kb)
Rights and permissions
About this article
Cite this article
Gonzalez, S., Lukacs-Kornek, V., Kuligowski, M. et al. Capture of influenza by medullary dendritic cells via SIGN-R1 is essential for humoral immunity in draining lymph nodes. Nat Immunol 11, 427–434 (2010). https://doi.org/10.1038/ni.1856
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.1856
This article is cited by
-
Applications of mannose-binding lectins and mannan glycoconjugates in nanomedicine
Journal of Nanoparticle Research (2022)
-
Dual-targeting nanoparticle vaccine elicits a therapeutic antibody response against chronic hepatitis B
Nature Nanotechnology (2020)
-
Extracellular bacterial lymphatic metastasis drives Streptococcus pyogenes systemic infection
Nature Communications (2020)
-
Age-related blunting of the phagocyte arsenal and its art of killing
Current Molecular Biology Reports (2020)
-
Protection against influenza infection requires early recognition by inflammatory dendritic cells through C-type lectin receptor SIGN-R1
Nature Microbiology (2019)