Abstract
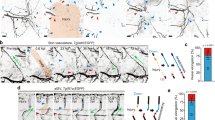
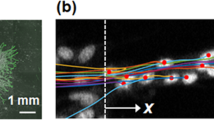
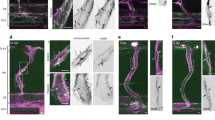
Formation of new vessels in granulation tissue during wound healing has been assumed to occur solely through sprouting angiogenesis. In contrast, we show here that neovascularization can be accomplished by nonangiogenic expansion of preexisting vessels. Using neovascularization models based on the chick chorioallantoic membrane and the healing mouse cornea, we found that tissue tension generated by activated fibroblasts or myofibroblasts during wound contraction mediated and directed translocation of the vasculature. These mechanical forces pulled vessels from the preexisting vascular bed as vascular loops with functional circulation that expanded as an integral part of the growing granulation tissue through vessel enlargement and elongation. Blockade of vascular endothelial growth factor receptor-2 confirmed that biomechanical forces were sufficient to mediate the initial vascular growth independently of endothelial sprouting or proliferation. The neovascular network was further remodeled by splitting, sprouting and regression of individual vessels. This model explains the rapid appearance of large functional vessels in granulation tissue during wound healing.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Dvorak, H.F. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N. Engl. J. Med. 315, 1650–1659 (1986).
Kalluri, R. & Zeisberg, M. Fibroblasts in cancer. Nat. Rev. Cancer 6, 392–401 (2006).
Singer, A.J. & Clark, R.A. Cutaneous wound healing. N. Engl. J. Med. 341, 738–746 (1999).
Gabbiani, G., Ryan, G.B. & Majne, G. Presence of modified fibroblasts in granulation tissue and their possible role in wound contraction. Experientia 27, 549–550 (1971).
Tomasek, J.J., Gabbiani, G., Hinz, B., Chaponnier, C. & Brown, R.A. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat. Rev. Mol. Cell Biol. 3, 349–363 (2002).
McClain, S.A. et al. Mesenchymal cell activation is the rate-limiting step of granulation tissue induction. Am. J. Pathol. 149, 1257–1270 (1996).
Serini, G. et al. The fibronectin domain ED-A is crucial for myofibroblastic phenotype induction by transforming growth factor-beta1. J. Cell Biol. 142, 873–881 (1998).
Gabbiani, G., Hirschel, B.J., Ryan, G.B., Statkov, P.R. & Majno, G. Granulation tissue as a contractile organ. A study of structure and function. J. Exp. Med. 135, 719–734 (1972).
Dudar, T.E. & Jain, R.K. Microcirculatory flow changes during tissue growth. Microvasc. Res. 25, 1–21 (1983).
Fukumura, D. et al. Paracrine regulation of angiogenesis and adipocyte differentiation during in vivo adipogenesis. Circ. Res. 93, e88–e97 (2003).
Gschwandtner, M.E. et al. Microcirculation is similar in ischemic and venous ulcers. Microvasc. Res. 62, 226–235 (2001).
Rendell, M.S. et al. The microvascular composition of the healing wound compared at skin sites with nutritive versus arteriovenous perfusion. J. Surg. Res. 80, 373–379 (1998).
Virag, J.I. & Murry, C.E. Myofibroblast and endothelial cell proliferation during murine myocardial infarct repair. Am. J. Pathol. 163, 2433–2440 (2003).
Thurston, G., Baluk, P. & McDonald, D.M. Determinants of endothelial cell phenotype in venules. Microcirculation 7, 67–80 (2000).
Lorena, D., Uchio, K., Costa, A.M. & Desmouliere, A. Normal scarring: importance of myofibroblasts. Wound Repair Regen. 10, 86–92 (2002).
Tonnesen, M.G., Feng, X. & Clark, R.A. Angiogenesis in wound healing. J. Investig. Dermatol. Symp. Proc. 5, 40–46 (2000).
Ausprunk, D.H. & Folkman, J. Migration and proliferation of endothelial cells in preformed and newly formed blood vessels during tumor angiogenesis. Microvasc. Res. 14, 53–65 (1977).
Clark, E.R. & Clark, E.L. Microscopic observations of the growth of blood capillaries in the living mammal. Am. J. Anat. 64, 251–299 (1939).
Burri, P.H. & Djonov, V. Intussusceptive angiogenesis—the alternative to capillary sprouting. Mol. Aspects Med. 23, S1–S27 (2002).
Dvorak, H.F. Rous-Whipple Award Lecture. How tumors make bad blood vessels and stroma. Am. J. Pathol. 162, 1747–1757 (2003).
Patan, S. et al. Vascular morphogenesis and remodeling in a model of tissue repair: blood vessel formation and growth in the ovarian pedicle after ovariectomy. Circ. Res. 89, 723–731 (2001).
Pettersson, A. et al. Heterogeneity of the angiogenic response induced in different normal adult tissues by vascular permeability factor/vascular endothelial growth factor. Lab. Invest. 80, 99–115 (2000).
Rogers, P.A. & Gargett, C.E. Endometrial angiogenesis. Angiogenesis 2, 287–294 (1998).
Kilarski, W.W., Jura, N. & Gerwins, P. Inactivation of Src family kinases inhibits angiogenesis in vivo: implications for a mechanism involving organization of the actin cytoskeleton. Exp. Cell Res. 291, 70–82 (2003).
Kilarski, W.W., Jura, N. & Gerwins, P. An ex vivo model for functional studies of myofibroblasts. Lab. Invest. 85, 643–654 (2005).
Lusimbo, W.S., Leighton, F.A. & Wobeser, G.A. Histology and ultrastructure of the chorioallantoic membrane of the mallard duck (Anas platyrhynchos). Anat. Rec. 259, 25–34 (2000).
Westerhausen, A., Kishi, J. & Prockop, D.J. Mutations that substitute serine for glycine alpha 1–598 and glycine alpha 1–631 in type I procollagen. The effects on thermal unfolding of the triple helix are position-specific and demonstrate that the protein unfolds through a series of cooperative blocks. J. Biol. Chem. 265, 13995–14000 (1990).
Woodley, D.T., Yamauchi, M., Wynn, K.C., Mechanic, G. & Briggaman, R.A. Collagen telopeptides (cross-linking sites) play a role in collagen gel lattice contraction. J. Invest. Dermatol. 97, 580–585 (1991).
Eastwood, M., Mudera, V.C., McGrouther, D.A. & Brown, R.A. Effect of precise mechanical loading on fibroblast populated collagen lattices: morphological changes. Cell Motil. Cytoskeleton 40, 13–21 (1998).
Lee, C.R., Grodzinsky, A.J. & Spector, M. The effects of cross-linking of collagen-glycosaminoglycan scaffolds on compressive stiffness, chondrocyte-mediated contraction, proliferation and biosynthesis. Biomaterials 22, 3145–3154 (2001).
Korff, T. & Augustin, H.G. Tensional forces in fibrillar extracellular matrices control directional capillary sprouting. J. Cell Sci. 112, 3249–3258 (1999).
Samolov, B. et al. Delayed inflammation-associated corneal neovascularization in MMP-2-deficient mice. Exp. Eye Res. 80, 159–166 (2005).
Morrison, J.C., Fraunfelder, F.W., Milne, S.T. & Moore, C.G. Limbal microvasculature of the rat eye. Invest. Ophthalmol. Vis. Sci. 36, 751–756 (1995).
Barnhill, R. & Ryan, T. Physical versus chemical factors in angiogenesis. Microvasc. Res. 23, 129–130 (1982).
Ingber, D.E. Mechanical signaling and the cellular response to extracellular matrix in angiogenesis and cardiovascular physiology. Circ. Res. 91, 877–887 (2002).
Mammoto, A. et al. A mechanosensitive transcriptional mechanism that controls angiogenesis. Nature 457, 1103–1108 (2009).
Clark, E.R. Studies on the growth of blood-vessels in the tail of the frog larva––by observation and experiment on the living animal. Am. J. Anat. 23, 37–88 (1918).
Risau, W. Mechanisms of angiogenesis. Nature 386, 671–674 (1997).
Dor, Y., Djonov, V. & Keshet, E. Making vascular networks in the adult: branching morphogenesis without a roadmap. Trends Cell Biol. 13, 131–136 (2003).
Djonov, V., Baum, O. & Burri, P.H. Vascular remodeling by intussusceptive angiogenesis. Cell Tissue Res. 314, 107–117 (2003).
Muthukkaruppan, V. & Auerbach, R. Angiogenesis in the mouse cornea. Science 205, 1416–1418 (1979).
Lockhart, A.C. et al. A clinical model of dermal wound angiogenesis. Wound Repair Regen. 11, 306–313 (2003).
Kumar, V., Abbas, A. & Nelson, F. Robbins & Cotran Pathologic Basis of Disease—Interactive Case Study. (Saunders, Philadelphia, 2004).
Gabbiani, G. The myofibroblast in wound healing and fibrocontractive diseases. J. Pathol. 200, 500–503 (2003).
Gerhardt, H. et al. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J. Cell Biol. 161, 1163–1177 (2003).
Tammela, T. et al. Blocking VEGFR-3 suppresses angiogenic sprouting and vascular network formation. Nature 454, 656–660 (2008).
Hlushchuk, R. et al. Tumor recovery by angiogenic switch from sprouting to intussusceptive angiogenesis after treatment with PTK787/ZK222584 or ionizing radiation. Am. J. Pathol. 173, 1173–1185 (2008).
Vosseler, S., Mirancea, N., Bohlen, P., Mueller, M.M. & Fusenig, N.E. Angiogenesis inhibition by vascular endothelial growth factor receptor-2 blockade reduces stromal matrix metalloproteinase expression, normalizes stromal tissue, and reverts epithelial tumor phenotype in surface heterotransplants. Cancer Res. 65, 1294–1305 (2005).
Tong, R.T. et al. Vascular normalization by vascular endothelial growth factor receptor 2 blockade induces a pressure gradient across the vasculature and improves drug penetration in tumors. Cancer Res. 64, 3731–3736 (2004).
Augustin, H.G. Antiangiogenic tumour therapy: will it work? Trends Pharmacol. Sci. 19, 216–222 (1998).
Junghans, B.M. & Collin, H.B. The limbal vascular response to corneal injury. An autoradiographic study. Cornea 8, 141–149 (1989).
Sholley, M.M., Ferguson, G.P., Seibel, H.R., Montour, J.L. & Wilson, J.D. Mechanisms of neovascularization. Vascular sprouting can occur without proliferation of endothelial cells. Lab. Invest. 51, 624–634 (1984).
Scherer, S.S. et al. The mechanism of action of the vacuum-assisted closure device. Plast. Reconstr. Surg. 122, 786–797 (2008).
Segers, V.F. & Lee, R.T. Stem-cell therapy for cardiac disease. Nature 451, 937–942 (2008).
Acknowledgements
We thank M. Aronsson for help with cornea experiments, J. Kilarska for help with image processing and analysis and K. Kullander and N. Rabe for help with microscopy of whole-mount corneas. We also thank M. Swartz for allowing us to use her laboratory to perform gel contraction and image analysis and A. Bikfalvi for comments. This study was supported by grants to P.G. from the Göran Gustafsson Foundation, the Swedish Cancer Foundation (project 4422-B04-O5XAB), the Swedish Research Council (project K2005-31X-15348-01A), the Children's Cancer Foundation of Sweden (project 04/037), Lions Cancer Research Foundation in Uppsala, the Magnus Bergvall Foundation, King Gustaf V's 80-Year Foundation and Uppsala Foundation for Medical Research, by grants to A.K. from the Crown Princess Margareta Foundation (KMA), the Edwin Jordan Foundation, Karolinska Institute research grants, Stiftelsen Synfrämjandets Forskningsfond, and Swedish Research Council (project 2006-13828-37891-37) and by grants to B.S. from St. Erik Eye Hospital Research Foundation, Sigvard and Marianne Bernadotte Research Foundation for Children's Eye Care and Stiftelsen Synfrämjandets Forskningsfond.
Author information
Authors and Affiliations
Contributions
W.W.K. was involved in the overall design of the study; performed most of the experiments involving the CAM, immunohistochemistry, in vitro gel contraction, whole-mount staining of cornea samples and statistical analysis; analyzed data; and wrote most of the manuscript. B.S. designed and performed the cornea experiments and statistical analysis, and she wrote portions of the manuscript and edited the manuscript. L.P. performed CAM experiments with PVA sponges, in vitro cell analysis, peptide inhibitor studies and cornea stainings. A.K. designed parts of cornea experiments and edited the manuscript. P.G. designed the overall study, analyzed data, and wrote and edited the manuscript.
Corresponding authors
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–11 and Supplementary Methods (PDF 3929 kb)
Rights and permissions
About this article
Cite this article
Kilarski, W., Samolov, B., Petersson, L. et al. Biomechanical regulation of blood vessel growth during tissue vascularization. Nat Med 15, 657–664 (2009). https://doi.org/10.1038/nm.1985
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.1985
This article is cited by
-
A perfusable, multifunctional epicardial device improves cardiac function and tissue repair
Nature Medicine (2021)
-
Adipose-derived mesenchymal stromal cells promote corneal wound healing by accelerating the clearance of neutrophils in cornea
Cell Death & Disease (2020)
-
FN-EDA mediates angiogenesis of hepatic fibrosis via integrin-VEGFR2 in a CD63 synergetic manner
Cell Death Discovery (2020)
-
Novel morphometric analysis of higher order structure of human radial peri-papillary capillaries: relevance to retinal perfusion efficiency and age
Scientific Reports (2019)
-
Critical Limb Ischemia Induces Remodeling of Skeletal Muscle Motor Unit, Myonuclear-, and Mitochondrial-Domains
Scientific Reports (2019)