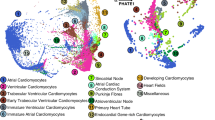
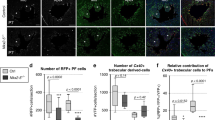
Abstract
Sympathetic innervation is critical for effective cardiac function. However, the developmental and regulatory mechanisms determining the density and patterning of cardiac sympathetic innervation remain unclear, as does the role of this innervation in arrhythmogenesis. Here we show that a neural chemorepellent, Sema3a, establishes cardiac sympathetic innervation patterning. Sema3a is abundantly expressed in the trabecular layer in early-stage embryos but is restricted to Purkinje fibers after birth, forming an epicardial-to-endocardial transmural sympathetic innervation patterning. Sema3a−/− mice lacked a cardiac sympathetic innervation gradient and exhibited stellate ganglia malformation, which led to marked sinus bradycardia due to sympathetic dysfunction. Cardiac-specific overexpression of Sema3a in transgenic mice (SemaTG) was associated with reduced sympathetic innervation and attenuation of the epicardial-to-endocardial innervation gradient. SemaTG mice demonstrated sudden death and susceptibility to ventricular tachycardia, due to catecholamine supersensitivity and prolongation of the action potential duration. We conclude that appropriate cardiac Sema3a expression is needed for sympathetic innervation patterning and is critical for heart rate control.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Accession codes
References
Crick, S.J. et al. Innervation of the human cardiac conduction system. A quantitative immunohistochemical and histochemical study. Circulation 89, 1697–1708 (1994).
Randall, W.C., Szentivanyi, M., Pace, J.B., Wechsler, J.S. & Kaye, M.P. Patterns of sympathetic nerve projections onto the canine heart. Circ. Res. 22, 315–323 (1968).
Ito, M. & Zipes, D.P. Efferent sympathetic and vagal innervation of the canine right ventricle. Circulation 90, 1459–1468 (1994).
Crick, S.J., Sheppard, M.N., Ho, S.Y. & Anderson, R.H. Localisation and quantitation of autonomic innervation in the porcine heart I: conduction system. J. Anat. 195, 341–357 (1999).
Chow, L.T., Chow, S.S., Anderson, R.H. & Gosling, J.A. Innervation of the human cardiac conduction system at birth. Br. Heart J. 69, 430–435 (1993).
Hansson, M., Kjorell, U. & Forsgren, S. Increased immunoexpression of atrial natriuretic peptide in the heart conduction system of the rat after cardiac sympathectomy. J. Mol. Cell. Cardiol. 30, 2047–2057 (1998).
Cao, J.M. et al. Nerve sprouting and sudden cardiac death. Circ. Res. 86, 816–821 (2000).
Cao, J.M. et al. Relationship between regional cardiac hyperinnervation and ventricular arrhythmia. Circulation 101, 1960–1969 (2000).
Opthof, T. et al. Dispersion of refractoriness in canine ventricular myocardium. Effects of sympathetic stimulation. Circ. Res. 68, 1204–1215 (1991).
Priori, S.G. & Corr, P.B. Mechanisms underlying early and delayed afterdepolarizations induced by catecholamines. Am. J. Physiol. 258, H1796–H1805 (1990).
Ieda, M. et al. Endothelin-1 regulates cardiac sympathetic innervation in the rodent heart by controlling nerve growth factor expression. J. Clin. Invest. 113, 876–884 (2004).
Kuruvilla, R. et al. A neurotrophin signaling cascade coordinates sympathetic neuron development through differential control of TrkA trafficking and retrograde signaling. Cell 118, 243–255 (2004).
Puschel, A.W., Adams, R.H. & Betz, H. Murine semaphorin D/collapsin is a member of a diverse gene family and creates domains inhibitory for axonal extension. Neuron 14, 941–948 (1995).
Kawasaki, T. et al. Requirement of neuropilin 1-mediated Sema3A signals in patterning of the sympathetic nervous system. Development 129, 671–680 (2002).
Tanelian, D.L., Barry, M.A., Johnston, S.A., Le, T. & Smith, G.M. Semaphorin III can repulse and inhibit adult sensory afferents in vivo. Nat. Med. 3, 1398–1401 (1997).
Taniguchi, M. et al. Disruption of semaphorin III/D gene causes severe abnormality in peripheral nerve projection. Neuron 19, 519–530 (1997).
Behar, O., Golden, J.A., Mashimo, H., Schoen, F.J. & Fishman, M.C. Semaphorin III is needed for normal patterning and growth of nerves, bones and heart. Nature 383, 525–528 (1996).
Pashmforoush, M. et al. Nkx2–5 pathways and congenital heart disease; loss of ventricular myocyte lineage specification leads to progressive cardiomyopathy and complete heart block. Cell 117, 373–386 (2004).
Tago, H., Kimura, H. & Maeda, T. Visualization of detailed acetylcholinesterase fiber and neuron staining in rat brain by a sensitive histochemical procedure. J. Histochem. Cytochem. 34, 1431–1438 (1986).
Kupershmidt, S. et al. Replacement by homologous recombination of the minK gene with lacZ reveals restriction of minK expression to the mouse cardiac conduction system. Circ. Res. 84, 146–152 (1999).
Shusterman, V. et al. Strain-specific patterns of autonomic nervous system activity and heart failure susceptibility in mice. Am. J. Physiol. Heart Circ. Physiol. 282, H2076–H2083 (2002).
Saba, S., London, B. & Ganz, L. Autonomic blockade unmasks maturational differences in rate-dependent atrioventricular nodal conduction and facilitation in the mouse. J. Cardiovasc. Electrophysiol. 14, 191–195 (2003).
Gulick, J., Subramaniam, A., Neumann, J. & Robbins, J. Isolation and characterization of the mouse cardiac myosin heavy chain genes. J. Biol. Chem. 266, 9180–9185 (1991).
Kitsukawa, T. et al. Neuropilin-semaphorin III/D-mediated chemorepulsive signals play a crucial role in peripheral nerve projection in mice. Neuron 19, 995–1005 (1997).
Xu, X.M. et al. The transmembrane protein semaphorin 6A repels embryonic sympathetic axons. J. Neurosci. 20, 2638–2648 (2000).
Wright, D.E., White, F.A., Gerfen, R.W., Silos-Santiago, I. & Snider, W.D. The guidance molecule semaphorin III is expressed in regions of spinal cord and periphery avoided by growing sensory axons. J. Comp. Neurol. 361, 321–333 (1995).
Tang, X.Q., Tanelian, D.L. & Smith, G.M. Semaphorin3A inhibits nerve growth factor-induced sprouting of nociceptive afferents in adult rat spinal cord. J. Neurosci. 24, 819–827 (2004).
Dae, M.W. et al. Heterogeneous sympathetic innervation in German shepherd dogs with inherited ventricular arrhythmia and sudden cardiac death. Circulation 96, 1337–1342 (1997).
Qu, J. & Robinson, R.B. Cardiac ion channel expression and regulation: the role of innervation. J. Mol. Cell. Cardiol. 37, 439–448 (2004).
Sosunov, E.A. et al. Long-term electrophysiological effects of regional cardiac sympathetic denervation of the neonatal dog. Cardiovasc. Res. 51, 659–669 (2001).
Stramba-Badiale, M., Lazzarotti, M. & Schwartz, P.J. Development of cardiac innervation, ventricular fibrillation, and sudden infant death syndrome. Am. J. Physiol. 263, H1514–H1522 (1992).
Chantranuwat, C. et al. Sudden, unexpected death in cardiac transplant recipients: an autopsy study. J. Heart Lung Transplant. 23, 683–689 (2004).
Gitler, A.D., Lu, M.M. & Epstein, J.A. PlexinD1 and semaphorin signaling are required in endothelial cells for cardiovascular development. Dev. Cell 7, 107–116 (2004).
Gu, C. et al. Neuropilin-1 conveys semaphorin and VEGF signaling during neural and cardiovascular development. Dev. Cell 5, 45–57 (2003).
Kuo, H.C. et al. A defect in the Kv channel-interacting protein 2 (KChIP2) gene leads to a complete loss of I(to) and confers susceptibility to ventricular tachycardia. Cell 107, 801–813 (2001).
Brunet, S. et al. Heterogeneous expression of repolarizing, voltage-gated K+ currents in adult mouse ventricles. J. Physiol. (Lond.) 559, 103–120 (2004).
Costantini, D.L. et al. The homeodomain transcription factor Irx5 establishes the mouse cardiac ventricular repolarization gradient. Cell 123, 347–358 (2005).
Takahashi, T. et al. Increased cardiac adenylyl cyclase expression is associated with increased survival after myocardial infarction. Circulation 114, 388–396 (2006).
Patel, T.D., Jackman, A., Rice, F.L., Kucera, J. & Snider, W.D. Development of sensory neurons in the absence of NGF/TrkA signaling in vivo. Neuron 25, 345–357 (2000).
Mohler, P.J. et al. Ankyrin-B mutation causes type 4 long-QT cardiac arrhythmia and sudden cardiac death. Nature 421, 634–639 (2003).
Ecker, P.M. et al. Effect of targeted deletions of beta1- and beta2-adrenergic-receptor subtypes on heart rate variability. Am. J. Physiol. Heart Circ. Physiol. 290, H192–H199 (2006).
Heart rate variability: standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology Circulation 93 1043 1065 (1996).
Wehrens, X.H. et al. Protection from cardiac arrhythmia through ryanodine receptor-stabilizing protein calstabin2. Science 304, 292–296 (2004).
Kannankeril, P.J. et al. Mice with the R176Q cardiac ryanodine receptor mutation exhibit catecholamine-induced ventricular tachycardia and cardiomyopathy. Proc. Natl. Acad. Sci. USA 103, 12179–12184 (2006).
Acknowledgements
We are grateful to J. Robbins (Cincinnati Children's Hospital) for the expression vector containing the α-myosin heavy chain promoter. We also thank Y. Tanimoto, Y. Miyake, H. Kawaguchi, E. Kobayashi and M. Nakamura for technical assistance. We are also grateful to the members of the Fukuda laboratory for their comments on the manuscript. This study was supported in part by research grants from the Ministry of Education, Culture, Sports, Science and Technology, Japan, and the Program for Promotion of Fundamental Studies in Health Sciences of the National Institute of Biomedical Innovation.
Author information
Authors and Affiliations
Contributions
M.I. designed the study, conducted all experiments, and wrote the manuscript. H.K. and K.K. conducted histochemical characterization. F.H. participated in subcloning. Y.I. conducted the growth cone collapse assay. M.T. provided Sema3a−/− mice and Sema3alacZ/lacZ mice. S.M., J.-K.L and I.K participated in and provided advice on the electrophysiology. K.M. and Y.T. participated in histochemical characterization. K.S. conducted the pronuclear microinjection. S.M. and M.S. participated in northern blotting and western blotting. S.O. provided advice on the experimental design. K.F. supported financially and supervised the whole project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
The time course of cardiac sympathetic innervation in developing hearts (PDF 718 kb)
Supplementary Fig. 2
The central conduction system in wild-type hearts (PDF 688 kb)
Supplementary Fig. 3
Sema3a expression and sympathetic innervation in SemaTG hearts (PDF 802 kb)
Supplementary Table 1
Sema3a–deficient mice display sinus bradycardia (PDF 1813 kb)
Supplementary Table 2
Electrophysiological data in WT and SemaTG mice (PDF 2024 kb)
Rights and permissions
About this article
Cite this article
Ieda, M., Kanazawa, H., Kimura, K. et al. Sema3a maintains normal heart rhythm through sympathetic innervation patterning. Nat Med 13, 604–612 (2007). https://doi.org/10.1038/nm1570
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm1570
This article is cited by
-
Treadmill exercise with nanoselenium supplementation affects the expression of Irisin/FNDC5 and semaphorin 3A in rats exposed to cigarette smoke extract
3 Biotech (2024)
-
Pathophysiological functions of semaphorins in the sympathetic nervous system
Inflammation and Regeneration (2023)
-
Semaphorin3f as a cardiomyocyte derived regulator of heart chamber development
Cell Communication and Signaling (2022)
-
Phenotype-tissue expression and exploration (PTEE) resource facilitates the choice of tissue for RNA-seq-based clinical genetics studies
BMC Genomics (2021)
-
The sympathetic nervous system in development and disease
Nature Reviews Neuroscience (2021)