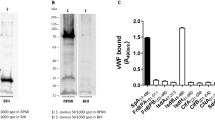
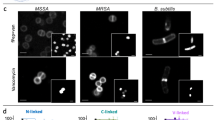
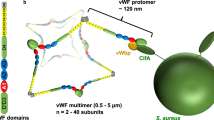
Abstract
Staphylococcus aureus is a human pathogen that secretes proteins that contribute to bacterial colonization. Here we describe the extracellular adherence protein (Eap) as a novel anti-inflammatory factor that inhibits host leukocyte recruitment. Due to its direct interactions with the host adhesive proteins intercellular adhesion molecule 1 (ICAM-1), fibrinogen or vitronectin, Eap disrupted β2-integrin and urokinase receptor–mediated leukocyte adhesion in vitro. Whereas Eap-expressing S. aureus induced a 2–3-fold lower neutrophil recruitment in bacterial peritonitis in mice as compared with an Eap-negative strain, isolated Eap prevented β2-integrin-dependent neutrophil recruitment in a mouse model of acute thioglycollate-induced peritonitis. Thus, the specific interactions with ICAM-1 and extracellular matrix proteins render Eap a potent anti-inflammatory factor, which may serve as a new therapeutic substance to block leukocyte extravasation in patients with hyperinflammatory pathologies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Lowy, F.D. Staphylococcus aureus infections. N. Engl. J. Med. 339, 520–532 (1998).
Brumfitt, W. & Hamilton-Miller, J. Methicillin-resistant Staphylococcus aureus. N. Engl. J. Med. 320, 1188–1196 (1989).
Waldvogel, F.A. New resistance in Staphylococcus aureus. N. Engl. J. Med. 340, 556–557 (1999).
Schlievert, P.M. Role of superantigens in human disease. J. Infect. Dis. 167, 997–1002 (1993).
Kim, J., Urban, R.G., Strominger, J.L. & Wiley, D.C. Toxic shock syndrome toxin-1 complexed with a class II major histocompatibility molecule HLA-DR1. Science 266, 1870–1874 (1994).
Jardetzky, T.S. et al. Three-dimensional structure of a human class II histocompatibility molecule complexed with superantigen. Nature 368, 711–718 (1994).
Bodén, M. & Flock, J.I. Fibrinogen-binding protein/clumping factor from Staphylococcus aureus. Infect. Immun. 57, 2358–2363 (1989).
Flock, J.I. et al. Cloning and expression of the gene for a fibronectin-binding protein from Staphylococcus aureus. EMBO J. 6, 2351–2357 (1987).
McDevitt, D., Francois, P., Vaudaux, P. & Foster, T.J. Molecular characterization of the clumping factor (fibrinogen receptor) of Staphylococcus aureus. Mol. Microbiol. 11, 237–248 (1994).
Park, P.W., Rosenbloom, J., Abrams, W.R., Rosenbloom, J. & Mecham, R.P. Molecular cloning and expression of the gene for elastin binding protein (ebpS) in Staphylococcus aureus. J. Biol. Chem. 271, 15803–15809 (1996).
Patti, J.M. et al. Molecular characterization and expression of a gene encoding a Staphylococcus aureus collagen adhesin. J. Biol. Chem. 267, 4766–4772 (1992).
Paulsson, M., Liang, O., Ascencio, F. & Wadström, T. Vitronectin binding surface proteins of Staphylococcus aureus. Zentbl. Bakteriol. 277, 54–64 (1992).
Flock, J.I. Extracellular-matrix-binding proteins as targets for the prevention of Staphylococcus aureus infections. Mol. Med. Today 12, 532–537 (1999).
Moreillon, P. et al. Role of Staphylococcus aureus coagulase and clumping factor in pathogenesis of experimental endocarditis. Infect. Immun. 63, 4738–4743 (1995).
Sinha, B. et al. Heterologously expressed Staphylococcus aureus fibronectin-binding proteins are sufficient for invasion of host cells. Infect. Immun. 68, 6871–6878 (2000).
Sinha, B. et al. Fibronectin-binding protein acts as Staphylococcus aureus invasin via fibronectin bridging to integrin α5β1. Cell. Microbiol. 1, 101–117 (1999).
Hendrix, H., Lindhout, T., Mertens, K., Engels, W. & Hemker, H.C. Activation of human prothrombin by stoichiometric levels of staphylocoagulase. J. Biol. Chem. 258, 3637–3644 (1983).
Sawai, T. et al. Role of coagulase in a murine model of hematogenous pulmonary infection induced by intravenous injection of Staphylococcus aureus enmeshed in agar beads. Infect. Immun. 65, 466–471 (1997).
Palma, M., Nozohoor, S., Schenning, T., Heimdahl, A. & Flock, J.I. Lack of the extracellular 19-kilodalton fibrinogen-binding protein from Staphylococcus aureus decreases virulence in experimental wound infection. Infect. Immun. 64, 5284–5289 (1996).
Jönsson, K., McDevitt, D., McGavin, M.H., Patti, J.M. & Höök, M. Staphylococcus aureus expresses a major histocompatibility complex class II analog. J. Biol. Chem. 270, 21457–21460 (1995).
McGavin, M.H., Krajewska-Pietrasik, D., Rydén, C. & Höök, M. Identification of a Staphylococcus aureus extracellular matrix-binding protein with broad specificity. Infect. Immun. 61, 2479–2485 (1993).
Palma, M., Haggar, A., Flock, J.I. Adherence of Staphylococcus aureus is enhanced by an endogenous secreted protein with broad binding activity. J. Bacteriol. 181, 2840–2845 (1999).
Springer, T.A. Traffic signals for lymphocyte recirculation and leukocyte emigration: The multistep paradigm. Cell 76, 301–314 (1994).
Carlos, T.M. & Harlan, J.M. Leukocyte-endothelial adhesion molecules. Blood 84, 2068–2101 (1994).
Plow, E.F., Haas, T.A., Zhang, L., Loftus, J. & Smith, J.W. Ligand binding to integrins. J. Biol. Chem. 275, 21785–21788 (2000).
Ossowski, L. & Aguirre-Ghiso, J.A. Urokinase-receptor and integrin partnership: Coordination of signaling for cell adhesion, migration and growth. Curr. Opin. Cell. Biol. 12, 613–620 (2000).
Preissner, K.T., Kanse, S.M. & May, A.E. Urokinase receptor: A molecular organizer in cellular communication. Curr. Opin. Cell. Biol. 12, 621–628 (2000).
Chapman, H.A., Wei, Y., Simon, D.I. & Waltz, D.A. Role of urokinase receptor and caveolin in regulation of integrin signaling. Thromb. Haemost. 82, 291–297 (1999).
Chavakis, T. et al. Different mechanisms define the antiadhesive function of high molecular weight kininogen in integrin- and urokinase receptor-dependent interactions. Blood 96, 514–522 (2000).
Chavakis, T., May, A.E., Preissner, K.T. & Kanse, S.M. Molecular mechanisms of zinc-dependent leukocyte adhesion involving the urokinase receptor and β2-integrins. Blood 93, 2976–2983 (1999).
May, A.E. et al. Urokinase receptor (CD87) regulates leukocyte recruitment via β2-integrins in vivo. J. Exp. Med. 188, 1029–1037 (1998).
Chavakis, T. et al. Regulation of leukocyte recruitment by polypeptides derived from high molecular weight kininogen. FASEB J. 15, 2365–2376 (2001).
Bosse, R. & Vestweber, D. Only simultaneous blocking of the L- and P-selectin completely inhibits neutrophil migration into mouse peritoneum. Eur. J. Immunol. 24, 3019–3024 (1994).
Borges, E. et al. The P-selectin glycoprotein ligand-1 is important for recruitment of neutrophils into inflamed mouse peritoneum. Blood 90, 1934–1942 (1997).
Hartleib, J. et al.. Protein A is the von Willebrand factor binding protein on Staphylococcus aureus. Blood 966, 2149–2156 (2000).
Hussain, M. et al. Insertional inactivation of Eap in staphylococcus aureus strain Newman confers reduced staphylococcal binding to fibroblasts. Infect. Immun. in press (2002).
Hoyer-Hansen, G., Behrendt, N., Ploug, M., Dano, K. & Preissner, K.T. The intact urokinase receptor is required for efficient vitronectin binding: Receptor cleavage prevents ligand interaction. FEBS Lett. 420, 79–85 (1997).
Stockmann, A., Hess, S., DeClerck, P., Timpl, R. & Preissner, K.T. Multimeric vitronectin: Identification and characterization of conformation-dependent self-association of the adhesive protein. J. Biol. Chem. 268, 22874–22882 (1993).
Palma, M., Wade, D., Flock, M. & Flock, J.I. Multiple binding sites in the interaction between fibrinogen and an extracellular fibrinogen binding protein from Staphylococcus aureus. J. Biol. Chem. 273, 13177–13181 (1998).
Hussain, M., Becker, K., von Eiff, C., Peters, G. & Herrmann, M. Analogs of Eap protein are conserved and prevalent in clinical Staphylococcus aureus isolates. Clin. Diagn. Lab. Immunol. 8, 1271–1276 (2001).
Hussain, M., Hastings, J.G.M. & White, P.J. A chemically defined medium for slime production by coagulase-negative staphylococci. J. Med. Microbiol. 34, 143–147 (1991).
Mizgerd, J.P. et al. Combinatorial requirements for adhesion molecules in mediating neutrophil emigration during bacterial peritonitis in mice. J. Leukoc. Biol. 64, 291–297 (1998).
Acknowledgements
We thank T. Schmidt-Wöll, S. Tannert-Otto and J. Weber for technical assistance; and G. Hoyer-Hansen, S. Bodary and D.B. Cines for reagents. This work was supported in part by grants from the Novartis Foundation for Therapeutical Research (to K.T.P. and T.C.), the Wilhelm–Sander Foundation (to K.T.P.) and by grants from the Deutsche Forschungsgemeinschaft (Specialized Research Centers 293 and 492 to M. Herrman and G.P.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Chavakis, T., Hussain, M., Kanse, S. et al. Staphylococcus aureus extracellular adherence protein serves as anti-inflammatory factor by inhibiting the recruitment of host leukocytes. Nat Med 8, 687–693 (2002). https://doi.org/10.1038/nm728
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm728
This article is cited by
-
Virulence factors and clonal diversity of Staphylococcus aureus in colonization and wound infection with emphasis on diabetic foot infection
European Journal of Clinical Microbiology & Infectious Diseases (2020)
-
Lactobacillus plantarum USM8613 Aids in Wound Healing and Suppresses Staphylococcus aureus Infection at Wound Sites
Probiotics and Antimicrobial Proteins (2020)
-
Interaction of host and Staphylococcus aureus protease-system regulates virulence and pathogenicity
Medical Microbiology and Immunology (2019)
-
Virulence analysis of Staphylococcus aureus in a rabbit model of infected full-thickness wound under negative pressure wound therapy
Antonie van Leeuwenhoek (2018)
-
The Staphylococcus aureus extracellular matrix protein (Emp) has a fibrous structure and binds to different extracellular matrices
Scientific Reports (2017)