Abstract
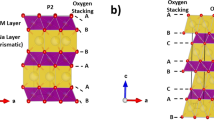
Sodium layered oxides NaxCoO2 form one of the most fascinating low-dimensional and strongly correlated systems; in particular P2–NaxCoO2 exhibits various single-phase domains with different Na+/vacancy patterns depending on the sodium concentration. Here we used sodium batteries to clearly depict the P2–NaxCoO2 phase diagram for x≥0.50. By coupling the electrochemical process with an in situ X-ray diffraction experiment, we identified the succession of single-phase or two-phase domains appearing on sodium intercalation with a rather good accuracy compared with previous studies. We reported new single-phase domains and we underlined the thermal instability of some ordered phases from an electrochemical study at various temperatures. As each phase is characterized by the position of its Fermi level versus the Na+/Na couple, we showed that the synthesis of each material, even in large amounts, can be carried out electrochemically. The physical properties of the as-prepared Na1/2CoO2 and Na2/3CoO2 ordered phases were characterized and compared. Electrochemical processes are confirmed to be an accurate route to precisely investigate in a continuous way such a complex system and provide a new way to synthesize materials with a very narrow existence range.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Dodero, M. & Déportes, C. Sur la préparation de ferrites, nickelites et cobaltites alcalins par électrolyse ignée. C. R. Acad. Sci; Paris 242, 2939–2941 (1956).
Déportes, C. Sur la préparation anodique des ferrites, nickelites et cobaltites alcalins par électrolyse ignée et l’étude de leurs propriétés. PhD Thesis, Univ. Grenoble (1958).
Delmas, C., Fouassier, C. & Hagenmuller, P. Structural classification and properties of the layered oxides. Physica B+C 99, 81–85 (1980).
Jansen, M. & Hoppe, R. Neue Oxocobaltate. Naturwissenschaften 59, 215 (1972).
Jansen, M. & Hoppe, R. Notiz zur kenntnis der oxocobaltate des natriums. Z. Anorg. Allg. Chem. 408, 104–106 (1974).
Fouassier, C., Matejka, G., Reau, J-M. & Hagenmuller, P. Sur de nouveaux bronzes oxygénés de formule NaxCoO2 (x<1). Le système cobalt-oxygène-sodium. J. Solid State Chem. 6, 532–537 (1973).
Braconnier, J-J., Delmas, C., Fouassier, C. & Hagenmuller, P. Comportement éléctrochimique des phases NaxCoO2 . Mater. Res. Bull. 15, 1797–1804 (1980).
Shaklette, L. W., Jow, T. R. & Townsend, L. Rechargeable electrodes from sodium cobalt bronzes. J. Electrochem. Soc. 135, 2669–2674 (1988).
Molenda, J., Delmas, C. & Hagenmuller, P. Electronic and electrochemical properties of NaxCoO2−y cathode. Solid State Ion. 9–10, 431–435 (1983).
Molenda, J., Delmas, C., Dordor, P. & Stoklosa, A. Transport properties of NaxCoO2−y . Solid State Ion. 12, 473–477 (1984).
Terasaki, I., Sasago, Y & Uchinokura, K. Large thermoelectric power in NaCo2O4 single crystals. Phys. Rev. B 56, R12685–R12687 (1997).
Takada, K. et al. Superconductivity in two-dimensional CoO2 layers. Nature 422, 53–55 (2003).
Lee, M. et al. Large enhancement of the thermopower in NaxCoO2 at high Na doping. Nature Mater. 5, 537–540 (2006).
Lee, M. et al. Enhancement of the thermopower in NaxCoO2 in the large-x regime (x≥0.75). Physica B 403, 1564–1568 (2008).
Zhang, P., Capaz, R., Cohen, M. & Louie, S. Theory of sodium ordering in NaxCoO2 . Phys. Rev. B 71, 153102 (2005).
Roger, M. et al. Patterning of sodium ions and the control of electrons in sodium cobaltate. Nature 445, 631–634 (2007).
Meng, Y., Hinuma, Y. & Ceder, G. An investigation of the sodium patterning in NaxCoO2 (0.5<x<1) by density functional theory methods. J. Chem. Phys. 128, 104708 (2008).
Blangero, M. et al. First experimental evidence of potassium ordering in layered K4Co7O14 . Inorg. Chem. 44, 9299–9304 (2005).
Carlier, D., Van der Ven, A., Delmas, C. & Ceder, G. First-principles investigation of phase stability in the O2–LiCoO2 system. Chem. Mater. 15, 2651–2660 (2003).
Huang, Q. et al. Low temperature phase transitions and crystal structure of Na0.5CoO2 . J. Phys. Condens. Matter 16, 5803–5814 (2004).
Mukhamedshin, I., Alloul, H., Collin, G. & Blanchard, N. Na NMR evidence for charge order and anomalous magnetism in NaxCoO2 . Phys. Rev. Lett. 93, 167601 (2004).
Alloul, H., Mukhamedshin, I., Collin, G. & Blanchard, N. Na atomic order, Co charge disproportionation and magnetism in NaxCoO2 for large Na contents. Europhys. Lett. 82, 17002 (2008).
Julien, M-H. et al. Electronic texture of the thermoelectric oxide Na0.75CoO2 . Phys. Rev. Lett. 100, 096405 (2008).
Platova, T., Mukhamedshin, I., Alloul, H., Dooglav, A. & Collin, G. NQR and X-ray investigation of the structure of Na2/3CoO2 compound. Phys. Rev. B 80, 224106 (2009).
Huang, Q. et al. Coupling between electronic and structural degrees of freedom in the triangular lattice conductor NaxCoO2 . Phys. Rev. B 70, 184110 (2004).
Chou, F. et al. Thermodynamic and transport measurements of superconducting Na0.3CoO2·1.3H2O single crystals prepared by electrochemical deintercalation. Phys. Rev. Lett. 92, 157004 (2004).
Chou, F., Abel, E., Cho, J. & Lee, Y. Electrochemical de-intercalation, oxygen non-stoichiometry, and crystal growth of NaxCoO2 . J. Phys. Chem. Solids 66, 155–160 (2005).
Shu, G. et al. Searching for stable Na-ordered phases in single-crystal samples of γ-NaxCoO2 . Phys. Rev. B 76, 184115 (2007).
Huang, F-T. et al. X-ray and electron diffraction studies of superlattices and long-range three-dimensional Na ordering in γ-NaxCoO2 (x=0.71 and 0.84). Phys. Rev. B 79, 014413 (2009).
Mendels, P. et al. Cascade of bulk magnetic phase transitions in NaxCoO2 as studied by muon spin rotation. Phys. Rev. Lett. 94, 136403 (2005).
Lang, G., Bobroff, J., Alloul, H., Collin, G. & Blanchard, N. Spin correlations and cobalt charge states: Phase diagram of sodium cobaltates. Phys. Rev. B 78, 155116 (2008).
Mendiboure, A., Delmas, C. & Hagenmuller, P. Electrochemical intercalation and deintercalation of NaxMnO2 bronzes. J. Solid State Chem. 57, 323–331 (1985).
Saadoune, I., Maazaz, A., Ménétrier, M. & Delmas, C. On the NaxNi0.6Co0.4O2 system: Physical and electrochemical studies. J. Solid State Chem. 122, 111–117 (1996).
Croguennec, L., Pouillerie, C. & Delmas, C. NiO2 obtained by electrochemical lithium deintercalation from lithium nickelate: Structural modifications. J. Electrochem. Soc. 147, 1314–1321 (2000).
Ohzuku, T., Ueda, A. & Yamamoto, N. Zero-strain insertion material of Li[Li1/3Ti5/3]O4 for rechargeable lithium cells. J. Electrochem. Soc. 142, 1431–1435 (1995).
Hinuma, Y., Meng, Y. & Ceder, G. Temperature-concentration phase diagram of P2–NaxCoO2 from first-principles calculations. Phys. Rev. B 77, 224111 (2008).
Zandbergen, H., Foo, M., Xu, Q., Kumar, V. & Cava, R.J. Sodium ion ordering in NaxCoO2: Electron diffraction study. Phys. Rev. B 70, 024101 (2004).
Huang, Q., Lynn, J., Toby, B., Foo, M-L. & Cava, R. J. Characterization of the structural transition in Na0.75CoO2 . J. Phys. Condens. Matter 17, 1831–1840 (2005).
Igarashi, D., Miyazaki, Y., Kajitani, T. & Yubuta, K. Disorder–order transitions in NaxCoO2(x∼0.58). Phys. Rev. B 78, 184112 (2008).
Balsys, R. & Davis, R. Refinement of the structure of Na0.74CoO2 using neutron powder diffraction. Solid State Ion. 93, 279–282 (1997).
Foo, M. et al. Charge ordering, commensurability, and metallicity in the phase diagram of the layered NaxCoO2 . Phys. Rev. Lett. 92, 247001 (2004).
Yang, H. X. et al. Phase separation, effects of magnetic field and high pressure on charge ordering in γ-Na0.5CoO2 . Mater. Chem. Phys. 94, 119–124 (2005).
Gasparovic, G. et al. Neutron scattering study of novel magnetic order in Na0.5CoO2 . Phys. Rev. Lett. 96, 046403 (2006).
Dordor, P., Marquestaut, E. & Villeneuve, G. Dispositif de mesures du pouvoir thermoélectrique sur des échantillons très résistants entre 4 et 300 K. Rev. Phys. Appl. 15, 1607–1612 (1980).
Kaurav, N., Wu, K. K., Kuo, Y. K., Shu, G. J. & Chou, F. C. Seebeck coefficient of NaxCoO2: Measurements and a narrow-band model. Phys. Rev. B 79, 075105 (2009).
Igarashi, D., Miyazaki, Y., Yubuta, K. & Kajitani, T. Precise control of Na content in the layered cobaltate γ-NaxCoO2 . J. Electron. Mater. 39, 1669–1673 (2010).
Acknowledgements
The authors thank S. Pechev for XRD measurement technical expertise, M. Pollet for fruitful discussions and S. Meng, M-H. Julien and B. J. Hwang for scientific exchanges. Financial support was provided by CNRS, ANR through the OCTE programme and Région Aquitaine. CEA is thanked for the scholarship to R.B.
Author information
Authors and Affiliations
Contributions
D.C. and C.D. planned the research. R.B. and D.C. carried out the experimental work. R.B., D.C. and C.D. analysed the data, and wrote and revised the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 1184 kb)
Rights and permissions
About this article
Cite this article
Berthelot, R., Carlier, D. & Delmas, C. Electrochemical investigation of the P2–NaxCoO2 phase diagram. Nature Mater 10, 74–80 (2011). https://doi.org/10.1038/nmat2920
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmat2920
This article is cited by
-
Fast-charge high-voltage layered cathodes for sodium-ion batteries
Nature Sustainability (2024)
-
Hydrothermal synthesis of polyimide-linked covalent organic frameworks towards ultrafast and stable cathodic sodium storage
Science China Chemistry (2024)
-
Competition between magnetic interactions and structural instabilities leading to itinerant frustration in the triangular lattice antiferromagnet LiCrSe2
Communications Materials (2023)
-
Formation and impact of nanoscopic oriented phase domains in electrochemical crystalline electrodes
Nature Materials (2023)
-
Copper diffusion related phase change and voltage decay in CuS cathode
Nano Research (2023)