Abstract
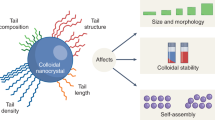
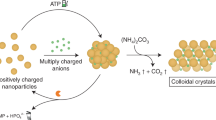
Microcrystals of desired sizes are important in a range of processes and materials, including controlled drug release1,2, production of pharmaceutics and food3,4, bio-5 and photocatalysis6, thin-film solar cells7 and antibacterial fabrics8. The growth of microcrystals can be controlled by a variety of agents, such as multivalent ions9, charged small molecules10, mixed cationic–anionic surfactants11,12, polyelectrolytes13,14 and other polymers15, micropatterned self-assembled monolayers16,17, proteins18 and also biological organisms during biomineralization19,20. However, the chief limitation of current approaches is that the growth-modifying agents are typically specific to the crystalizing material. Here, we show that oppositely charged nanoparticles can function as universal surfactants that control the growth and stability of microcrystals of monovalent or multivalent inorganic salts, and of charged organic molecules. We also show that the solubility of the microcrystals can be further tuned by varying the thickness of the nanoparticle surfactant layers and by reinforcing these layers with dithiol crosslinks.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Change history
20 January 2012
In the version of this Letter originally published online, the name of the penultimate author was spelt incorrectly; it should have read Shuangbing Han. This error has been corrected in all versions of the Letter.
References
Ai, H., Jones, S. A., de Villiers, M. M. & Lvov, Y. M. Nano-encapsulation of furosemide microcrystals for controlled drug release. J. Control. Release 86, 59–68 (2003).
Qiu, X. P., Leporatti, S., Donath, E. & Mohwald, H. Studies on the drug release properties of polysaccharide multilayers encapsulated ibuprofen microparticles. Langmuir 17, 5375–5380 (2001).
Ek, R., Wormald, P., Ostelius, J., Iversen, T. & Nystrom, C. Crystallinity index of microcrystalline cellulose particles compressed into tablets. Int. J. Pharm. 125, 257–264 (1995).
Bodmeier, R. Tableting of coated pellets. Eur. J. Pharm. Biopharm. 43, 1–8 (1997).
Kreiner, M., Moore, B. D. & Parker, M. C. Enzyme-coated micro-crystals: A 1-step method for high activity biocatalyst preparation. Chem. Commun. 41, 1096–1097 (2001).
Pradhan, A. R., Macnaughtan, M. A. & Raftery, D. Zeolite-coated optical microfibers for intrazeolite photocatalysis studied by in situ solid-state NMR. J. Am. Chem. Soc. 122, 404–405 (2000).
Rech, B. et al. Microcrystalline silicon for large area thin film solar cells. Thin Solid Films 427, 157–165 (2003).
Lee, G. S., Lee, Y. J., Ha, K. & Yoon, K. B. Preparation of flexible zeolite-tethering vegetable fibers. Adv. Mater. 13, 1491–1495 (2001).
Kubota, N., Otosaka, H., Doki, N., Yokota, M. & Sato, A. Effect of lead(II) impurity on the growth of sodium chloride crystals. J. Cryst. Growth 220, 135–139 (2000).
Mann, S., Didymus, J. M., Sanderson, N. P., Heywood, B. R. & Samper, E. J. A. Morphological influence of functionalized and non-functionalized- α,ω-dicarboxylates on calcite crystallization. J. Chem. Soc. Faraday Trans. 86, 1873–1880 (1990).
Jing, S. Y. et al. Synthesis of octahedral Cu2O microcrystals assisted with mixed cationic/anionic surfactants. Mater. Lett. 61, 2281–2283 (2007).
Tian, G. R., Sun, S. X., Song, X. Y., Zhao, W. & You, T. Cooperative role of dual surfactants in synthesis of single crystal BaMoO4 microcrystals. Mater. Technol. 24, 92–96 (2009).
Xiao, J. J., Kan, A. T. & Tomson, M. B. Prediction of BaSO4 precipitation in the presence and absence of a polymeric inhibitor: Phosphino-polycarboxylic acid. Langmuir 17, 4668–4673 (2001).
Oner, M. & Akyol, E. Inhibition of calcium oxalate monohydrate crystal growth using polyelectrolytes. J. Cryst. Growth 307, 137–144 (2007).
Yang, J. H., Qi, L. M., Zhang, D. B., Ma, J. M. & Cheng, H. M. Dextran-controlled crystallization of silver microcrystals with novel morphologies. Cryst. Growth Des. 4, 1371–1375 (2004).
Aizenberg, J., Black, A. J. & Whitesides, G. H. Oriented growth of calcite controlled by self-assembled monolayers of functionalized alkanethiols supported on gold and silver. J. Am. Chem. Soc. 121, 4500–4509 (1999).
Aizenberg, J., Black, A. J. & Whitesides, G. M. Control of crystal nucleation by patterned self-assembled monolayers. Nature 398, 495–498 (1999).
Yeh, Y. & Feeney, R. E. Antifreeze proteins: Structures and mechanisms of function. Chem. Rev. 96, 601–617 (1996).
Lowenstam, H. A. Minerals formed by organisms. Science 211, 1126–1131 (1981).
Addadi, L. & Weiner, S. Control and design principles in biological mineralization. Angew. Chem. Int. Edn. 31, 153–169 (1992).
Gref, R. et al. The controlled intravenous delivery of drugs using PEG-coated sterically stabilized nanospheres. Adv. Drug Deliv. Rev. 16, 215–233 (1995).
Panyam, J. & Labhasetwar, V. Biodegradable nanoparticles for drug and gene delivery to cells and tissue. Adv. Drug Deliv. Rev. 55, 329–347 (2003).
Kalsin, A. M., Kowalczyk, B., Smoukov, S. K., Klajn, R. & Grzybowski, B. A. Ionic-like behavior of oppositely charged nanoparticles. J. Am. Chem. Soc. 128, 15046–15047 (2006).
Kalsin, A. M., Kowalczyk, B., Wesson, P., Paszewski, M. & Grzybowski, B. A. Studying the thermodynamics of surface reactions on nanoparticles by electrostatic titrations. J. Am. Chem. Soc. 129, 6664–6665 (2007).
Kalsin, A. M. et al. Electrostatic self-assembly of binary nanoparticle crystals with a diamond-like lattice. Science 312, 420–424 (2006).
Kowalczyk, B. et al. Size selection during crystallization of oppositely charged nanoparticles. Chem. Eur. J. 15, 2032–2035 (2009).
Templeton, A. C., Zamborini, F. P., Wuelfing, W. P. & Murray, R. W. Controlled and reversible formation of nanoparticle aggregates and films using Cu2+-carboxylate chemistry. Langmuir 16, 6682–6688 (2000).
Wang, D., Tejerina, B., Lagzi, I., Kowalczyk, B. & Grzybowski, B. A. Bridging interactions and selective nanoparticle aggregation mediated by monovalent cations. ACS Nano 5, 530–536 (2011).
Tretiakov, K. V. et al. Mechanism of the cooperative adsorption of oppositely charged nanoparticles. J. Phys. Chem. A 113, 3799–3803 (2009).
Bishop, K. J. M., Wilmer, C. E., Soh, S. & Grzybowski, B. A. Nanoscale forces and their uses in self-assembly. Small 5, 1600–1630 (2009).
Smoukov, S. K., Bishop, K. J. M., Kowalczyk, B., Kalsin, A. M. & Grzybowski, B. A. Electrostatically ‘patchy’ coatings via cooperative adsorption of charged nanoparticles. J. Am. Chem. Soc. 129, 15623–15630 (2007).
Jones, F., Richmond, W. R. & Rohl, A. L. Molecular modeling of phosphonate molecules onto barium sulfate terraced surfaces. J. Phys. Chem. B 110, 7414–7424 (2006).
Kowalczyk, B., Lagzi, I. & Grzybowski, B. A. ‘Nanoarmoured’ droplets of different shapes formed by interfacial self-assembly and crosslinking of metal nanoparticles. Nanoscale 2, 2366–2369 (2010).
Acknowledgements
This work was supported by the Non-equilibrium Energy Research Center, which is an Energy Frontier Research Center funded by the US Department of Energy, Office of Science, Office of Basic Energy Sciences under award number DE-SC0000989.
Author information
Authors and Affiliations
Contributions
B.K. carried out the majority of experiments and imaging studies, and produced the figures; K.J.M.B. developed theoretical models; I.L. and D.W. helped with NP synthesis; Y.W. and S.H. collected and interpreted crystallographic data; B.A.G. conceived the project and wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 1212 kb)
Rights and permissions
About this article
Cite this article
Kowalczyk, B., Bishop, K., Lagzi, I. et al. Charged nanoparticles as supramolecular surfactants for controlling the growth and stability of microcrystals. Nature Mater 11, 227–232 (2012). https://doi.org/10.1038/nmat3202
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmat3202
This article is cited by
-
Nanoparticle surfactants for kinetically arrested photoactive assemblies to track light-induced electron transfer
Nature Nanotechnology (2021)
-
Self-assembled Cube-like Copper Oxide Derived from a Metal-Organic Framework as a High-Performance Electrochemical Supercapacitive Electrode Material
Scientific Reports (2019)
-
A Novel Method to Achieve Grain Refinement in Aluminum
Metallurgical and Materials Transactions A (2016)
-
Nanoscale surface chemistry directs the tunable assembly of silver octahedra into three two-dimensional plasmonic superlattices
Nature Communications (2015)
-
Copper Salts Mediated Morphological Transformation of Cu2O from Cubes to Hierarchical Flower-like or Microspheres and Their Supercapacitors Performances
Scientific Reports (2015)