Abstract
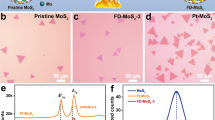
As a promising non-precious catalyst for the hydrogen evolution reaction (HER; refs 1,2,3,4,5), molybdenum disulphide (MoS2) is known to contain active edge sites and an inert basal plane1,6,7,8. Activating the MoS2 basal plane could further enhance its HER activity but is not often a strategy for doing so. Herein, we report the first activation and optimization of the basal plane of monolayer 2H-MoS2 for HER by introducing sulphur (S) vacancies and strain. Our theoretical and experimental results show that the S-vacancies are new catalytic sites in the basal plane, where gap states around the Fermi level allow hydrogen to bind directly to exposed Mo atoms. The hydrogen adsorption free energy (ΔGH) can be further manipulated by straining the surface with S-vacancies, which fine-tunes the catalytic activity. Proper combinations of S-vacancy and strain yield the optimal ΔGH = 0 eV, which allows us to achieve the highest intrinsic HER activity among molybdenum-sulphide-based catalysts.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Change history
18 November 2015
In the version of this Letter originally published, the topmost tick labels on the y axis in Figs 1b and 1c were incorrect and should have read 2.0. This is now correct in all versions of the Letter.
19 January 2016
In the version of this Letter originally published, the name of one of the co-authors was misspelled and should have been 'Hyun Soo Han'. This has been corrected in the online versions after print.
References
Hinnemann, B. et al. Biomimetic hydrogen evolution: MoS2 nanoparticles as catalyst for hydrogen evolution. J. Am. Chem. Soc. 127, 5308–5309 (2005).
Greeley, J. et al. Computational high-throughput screening of electrocatalytic materials for hydrogen evolution. Nature Mater. 5, 909–913 (2006).
Merki, D. & Hu, X. Recent developments of molybdenum and tungsten sulfides as hydrogen evolution catalysts. Energy Environ. Sci. 4, 3878–3888 (2011).
Chen, W.-F., Muckerman, J. T. & Fujita, E. Recent developments intransition metal carbides and nitrides as hydrogen evolution electrocatalysts. Chem. Commun. 49, 8896–8909 (2013).
Benck, J. D., Hellstern, T. R., Kibsgaard, J., Chakthranont, P. & Jaramillo, T. F. Catalyzing the hydrogen evolution reaction (HER) with molybdenum sulfide nanomaterials. ACS Catal. 4, 3957–3971 (2014).
Jaramillo, T. F. et al. Identification of active edge sites for electrochemical H2 evolution from MoS2 nanocatalysts. Science 317, 100–102 (2007).
Li, Y. et al. MoS2 nanoparticles grown on graphene: An advanced catalyst for the hydrogen evolution reaction. J. Am. Chem. Soc. 133, 7296–7299 (2011).
Chang, K. et al. MoS2/graphene cocatalyst for efficient photocatalytic H2 evolution under visible light irradiation. ACS Nano 8, 7078–7087 (2014).
Kong, D. et al. Synthesis of MoS2 and MoSe2 films with vertically aligned layers. Nano Lett. 13, 1341–1347 (2013).
Chen, Z. et al. Core–shell MoO3–MoS2 nanowires for hydrogen evolution: A functional design for electrocatalytic materials. Nano Lett. 11, 4168–4175 (2011).
Xie, J. et al. Defect-rich MoS2 ultrathin nanosheets with additional active edge sites for enhanced electrocatalytic hydrogen evolution. Adv. Mater. 25, 5807–5813 (2013).
Kibsgaard, J., Chen, Z., Reinecke, B. N. & Jaramillo, T. F. Engineering the surface structure of MoS2 to preferentially expose active edge sites for electrocatalysis. Nature Mater. 11, 963–969 (2012).
Bonde, J., Moses, P. G., Jaramillo, T. F., Nørskov, J. K. & Chorkendorff, I. Hydrogen evolution on nano-particulate transition metal sulfides. Faraday Discuss. 140, 219–231 (2009).
Wang, H. et al. Transition-metal doped edge sites in vertically aligned MoS2 catalysts for enhanced hydrogen evolution. Nano Res. 8, 566–575 (2015).
Lukowski, M. A. et al. Enhanced hydrogen evolution catalysis from chemically exfoliated metallic MoS2 nanosheets. J. Am. Chem. Soc. 135, 10274–10277 (2013).
Voiry, D. et al. Conducting MoS2 nanosheets as catalysts for hydrogen evolution reaction. Nano Lett. 13, 6222–6227 (2013).
Nørskov, J. K. et al. Universality in heterogeneous catalysis. J. Catal. 209, 275–278 (2002).
Nørskov, J. K. et al. Trends in the exchange current for hydrogen evolution. J. Electrochem. Soc. 152, J23–J26 (2005).
Greeley, J. & Mavrikakis, M. Alloy catalysts designed from first principles. Nature Mater. 3, 810–815 (2004).
Michalsky, R., Zhang, Y.-J. & Peterson, A. A. Trends in the hydrogen evolution activity of metal carbide catalysts. ACS Catal. 4, 1274–1278 (2014).
Tsai, C., Abild-Pedersen, F. & Nørskov, J. K. Tuning the MoS2 edge-site activity for hydrogen evolution via support interactions. Nano Lett. 14, 1381–1387 (2014).
Tsai, C., Chan, K., Abild-Pedersen, F. & Nørskov, J. K. Theoretical insights into the hydrogen evolution activity of layered transition metal dichalcogenides. Surf. Sci. 640, 133–140 (2015).
Strasser, P. et al. Lattice-strain control of the activity in dealloyed core-shell fuel cell catalysts. Nature Chem. 2, 454–460 (2010).
Voiry, D. et al. Enhanced catalytic activity in strained chemically exfoliated WS2 nanosheets for hydrogen evolution. Nature Mater. 12, 850–855 (2013).
Yildiz, B. “Stretching” the energy landscape of oxide-effects on electrocatalysis diffusion. MRS Bull. 39, 147–156 (2014).
Li, H. et al. Optoelectronic crystal of artificial atoms in strain-textured MoS2 . Nature Commun. 6, 7381 (2015).
Quan, M. et al. Controlled argon beam-induced desulfurization of monolayer molybdenum disulfide. J. Phys. Condens. Matter. 25, 252201 (2013).
Kibsgaard, J., Jaramillo, T. F. & Besenbacher, F. Building an appropriate active-site motif into a hydrogen-evolution catalyst with thiomolybdate [Mo3S13]2− clusters. Nature Chem. 6, 248–253 (2014).
Jaramillo, T. F. et al. Hydrogen evolution on supported incomplete cubane-type [Mo3S4]4+ electrocatalysts. J. Phys. Chem. C 112, 17492–17498 (2008).
Remskar, M. et al. Self-assembly of subnanometer-diameter single-wall MoS2 nanotubes. Science 292, 479–481 (2001).
Wellendorff, J. et al. Density functionals for surface science: Exchange-correlation model development with Bayesian error estimation. Phys. Rev. B 85, 235149 (2012).
Gohda, Y., Schnur, S. & Groß, A. Influence of water on elementary reaction steps in electrocatalysis. Faraday Discuss. 140, 233–244 (2009).
Kuisma, M., Ojanen, J., Enkovaara, J. & Rantala, T. T. Kohn–Sham potential with discontinuity for band gap materials. Phys. Rev. B 82, 115106 (2010).
Najmaei, S. et al. Vapour phase growth and grain boundary structure of molybdenum disulphide atomic layers. Nature Mater. 12, 754–759 (2013).
Acknowledgements
This work was financially supported by the 2013 Global Research Outreach (GRO) Program (Award number IC2012-1318) of the Samsung Advanced Institute of Technology (SAIT) and Samsung R&D Center America, Silicon Valley (SRA-SV) under the supervision of D. Bera and A. Radspieler Jr. We acknowledge support from the Center on Nanostructuring for Efficient Energy Conversion (CNEEC) at Stanford University, an Energy Frontier Research Center funded by the US Department of Energy, Office of Basic Energy Sciences under award number DE-SC0001060. F.A.-P. and J.K.N. acknowledge financial support from the US Department of Energy (DOE), Office of Basic Energy Sciences to the SUNCAT Center for Interface Science and Catalysis. A.W.C., A.H.F. and H.C.M. acknowledge financial support from the US Department of Energy, Office of Basic Energy Sciences, Division of Materials Sciences and Engineering, under contract DE-AC02-76SF00515. C.T. acknowledges support from the National Science Foundation Graduate Research Fellowship Program (GRFP) Grant DGE-114747.
Author information
Authors and Affiliations
Contributions
H.L. and X.Z. conceived the idea and designed the experiments. H.L. performed material growth, electrode fabrication and electrochemical measurements. C.T., J.K.N. and F.A.-P. carried out the theoretical calculations. A.L.K. conducted the TEM characterization. L.C. performed the XPS measurement. A.W.C., A.H.F. and H.C.M. conducted the STM/STS measurements. J.Z. and H.S.H. assisted with the electrochemical measurements. H.L., C.T. and X.Z. wrote the manuscript, and all authors discussed the results and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 6385 kb)
Rights and permissions
About this article
Cite this article
Li, H., Tsai, C., Koh, A. et al. Activating and optimizing MoS2 basal planes for hydrogen evolution through the formation of strained sulphur vacancies. Nature Mater 15, 48–53 (2016). https://doi.org/10.1038/nmat4465
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmat4465
This article is cited by
-
Single-atomic activation on ZnIn2S4 basal planes boosts photocatalytic hydrogen evolution
Nano Research (2024)
-
In-situ atomic level observation of the strain response of graphene lattice
Scientific Reports (2023)
-
Electrical spectroscopy of defect states and their hybridization in monolayer MoS2
Nature Communications (2023)
-
Strain enhances the activity of molecular electrocatalysts via carbon nanotube supports
Nature Catalysis (2023)
-
On-chip electrocatalytic microdevices
Nature Protocols (2023)