Abstract
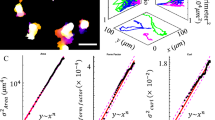
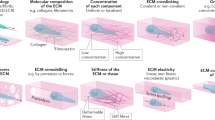
Living cells and the extracellular matrix (ECM) can exhibit complex interactions that define key developmental, physiological and pathological processes. Here, we report a new type of directed migration—which we term ‘topotaxis’—guided by the gradient of the nanoscale topographic features in the cells’ ECM environment. We show that the direction of topotaxis is reflective of the effective cell stiffness, and that it depends on the balance of the ECM-triggered signalling pathways PI(3)K–Akt and ROCK–MLCK. In melanoma cancer cells, this balance can be altered by different ECM inputs, pharmacological perturbations or genetic alterations, particularly a loss of PTEN in aggressive melanoma cells. We conclude that topotaxis is a product of the material properties of cells and the surrounding ECM, and propose that the invasive capacity of many cancers may depend broadly on topotactic responses, providing a potentially attractive mechanism for controlling invasive and metastatic behaviour.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Change history
22 March 2016
In the version of the Article originally published, the original title omitted a word and should have read 'Directed migration of cancer cells guided by the graded texture of the underlying matrix'. This has been corrected in all versions of the Article.
References
Devreotes, P. & Janetopoulos, C. Eukaryotic chemotaxis: distinctions between directional sensing and polarization. J. Biol. Chem. 278, 20445–20448 (2003).
McCarthy, J. B. & Furcht, L. T. Laminin and fibronectin promote the haptotactic migration of B16 mouse melanoma cells in vitro. J. Cell Biol. 98, 1474–1480 (1984).
Aznavoorian, S., Stracke, M. L., Krutzsch, H., Schiffmann, E. & Liotta, L. A. Signal transduction for chemotaxis and haptotaxis by matrix molecules in tumor cells. J. Cell Biol. 110, 1427–1438 (1990).
Lo, C. M., Wang, H. B., Dembo, M. & Wang, Y. L. Cell movement is guided by the rigidity of the substrate. Biophys. J. 79, 144–152 (2000).
Li, S., Huang, N. F. & Hsu, S. Mechanotransduction in endothelial cell migration. J. Cell Biochem. 96, 1110–1126 (2005).
Kim, D. H., Provenzano, P. P., Smith, C. L. & Levchenko, A. Matrix nanotopography as a regulator of cell function. J. Cell Biol. 197, 351–360 (2012).
Dalby, M. J., Riehle, M. O., Yarwood, S. J., Wilkinson, C. D. & Curtis, A. S. Nucleus alignment and cell signaling in fibroblasts: response to a micro-grooved topography. Exp. Cell Res. 284, 274–282 (2003).
Diehl, K. A., Foley, J. D., Nealey, P. F. & Murphy, C. J. Nanoscale topography modulates corneal epithelial cell migration. J. Biomed. Mater. Res. A 75, 603–611 (2005).
Kaiser, J. P., Reinmann, A. & Bruinink, A. The effect of topographic characteristics on cell migration velocity. Biomaterials 27, 5230–5241 (2006).
Teixeira, A. I. et al. The effect of environmental factors on the response of human corneal epithelial cells to nanoscale substrate topography. Biomaterials 27, 3945–3954 (2006).
Kim, D. H. et al. Mechanosensitivity of fibroblast cell shape and movement to anisotropic substratum topography gradients. Biomaterials 30, 5433–5444 (2009).
Kim, D. H. et al. Guided cell migration on microtextured substrates with variable local density and anisotropy. Adv. Funct. Mater. 19, 1579–1586 (2009).
Sochol, R. D., Higa, A. T., Janairo, R. R. R., Li, S. & Lin, L. W. Unidirectional mechanical cellular stimuli via micropost array gradients. Soft Matter 7, 4606–4609 (2011).
Miller, A. J. & Mihm, M. C. Jr Melanoma. N. Engl. J. Med. 355, 51–65 (2006).
Kessenbrock, K., Plaks, V. & Werb, Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell 141, 52–67 (2010).
Mott, J. D. & Werb, Z. Regulation of matrix biology by matrix metalloproteinases. Curr. Opin. Cell Biol. 16, 558–564 (2004).
Gaggioli, C. et al. Tumor-derived fibronectin is involved in melanoma cell invasion and regulated by V600E B-Raf signaling pathway. J. Invest. Dermatol. 127, 400–410 (2007).
Kaariainen, E. et al. Switch to an invasive growth phase in melanoma is associated with tenascin-C, fibronectin, and procollagen-I forming specific channel structures for invasion. J. Pathol. 210, 181–191 (2006).
Ushiki, T. Collagen fibers, reticular fibers and elastic fibers. A comprehensive understanding from a morphological viewpoint. Arch. Histol. Cytol. 65, 109–126 (2002).
Smith, L. A. & Ma, P. X. Nano-fibrous scaffolds for tissue engineering. Colloids Surf. B Biointerfaces 39, 125–131 (2004).
Chhabra, E. S. & Higgs, H. N. The many faces of actin: matching assembly factors with cellular structures. Nature Cell Biol. 9, 1110–1121 (2007).
Charras, G. T., Hu, C. K., Coughlin, M. & Mitchison, T. J. Reassembly of contractile actin cortex in cell blebs. J. Cell Biol. 175, 477–490 (2006).
Morone, N. et al. Three-dimensional reconstruction of the membrane skeleton at the plasma membrane interface by electron tomography. J. Cell Biol. 174, 851–862 (2006).
Gilden, J. & Krummel, M. F. Control of cortical rigidity by the cytoskeleton: emerging roles for septins. Cytoskeleton 67, 477–486 (2010).
Thiery, J. P., Acloque, H., Huang, R. Y. & Nieto, M. A. Epithelial-mesenchymal transitions in development and disease. Cell 139, 871–890 (2009).
Fabry, B. et al. Scaling the microrheology of living cells. Phys. Rev. Lett. 87, 148102 (2001).
An, S. S., Laudadio, R. E., Lai, J., Rogers, R. A. & Fredberg, J. J. Stiffness changes in cultured airway smooth muscle cells. Am. J. Physiol. Cell Physiol. 283, C792–C801 (2002).
Wettschureck, N. & Offermanns, S. Rho/Rho-kinase mediated signaling in physiology and pathophysiology. J. Mol. Med. 80, 629–638 (2002).
Riento, K. & Ridley, A. J. Rocks: multifunctional kinases in cell behaviour. Nature Rev. Mol. Cell Biol. 4, 446–456 (2003).
Wilkinson, S., Paterson, H. F. & Marshall, C. J. Cdc42–MRCK and Rho–ROCK signalling cooperate in myosin phosphorylation and cell invasion. Nature Cell Biol. 7, 255–261 (2005).
Innocenti, M. et al. Phosphoinositide 3-kinase activates Rac by entering in a complex with Eps8, Abi1, and Sos-1. J. Cell Biol. 160, 17–23 (2003).
Welch, H. C., Coadwell, W. J., Stephens, L. R. & Hawkins, P. T. Phosphoinositide 3-kinase-dependent activation of Rac. FEBS Lett. 546, 93–97 (2003).
Levine, H., Kessler, D. A. & Rappel, W. J. Directional sensing in eukaryotic chemotaxis: a balanced inactivation model. Proc. Natl Acad. Sci. USA 103, 9761–9766 (2006).
Janetopoulos, C. & Firtel, R. A. Directional sensing during chemotaxis. FEBS Lett. 582, 2075–2085 (2008).
Yamada, K. M. & Araki, M. Tumor suppressor PTEN: modulator of cell signaling, growth, migration and apoptosis. J. Cell Sci. 114, 2375–2382 (2001).
Wu, H., Goel, V. & Haluska, F. G. PTEN signaling pathways in melanoma. Oncogene 22, 3113–3122 (2003).
Levchenko, A. & Iglesias, P. A. Models of eukaryotic gradient sensing: application to chemotaxis of amoebae and neutrophils. Biophys. J. 82, 50–63 (2002).
Nogueira, C. et al. Cooperative interactions of PTEN deficiency and RAS activation in melanoma metastasis. Oncogene 29, 6222–6232 (2010).
Hwang, P. H. et al. Suppression of tumorigenicity and metastasis in B16F10 cells by PTEN/MMAC1/TEP1 gene. Cancer Lett. 172, 83–91 (2001).
Jeon, H. et al. Directing cell migration and organization via nanocrater-patterned cell-repellent interfaces. Nature Mater. 14, 918–923 (2015).
Saez, A., Ghibaudo, M., Buguin, A., Silberzan, P. & Ladoux, B. Rigidity-driven growth and migration of epithelial cells on microstructured anisotropic substrates. Proc. Natl Acad. Sci. USA 104, 8281–8286 (2007).
Ghassemi, S. et al. Cells test substrate rigidity by local contractions on submicrometer pillars. Proc. Natl Acad. Sci. USA 109, 5328–5333 (2012).
Kim, P. et al. Fabrication of nanostructures of polyethylene glycol for applications to protein adsorption and cell adhesion. Nanotechnology 16, 2420–2426 (2005).
Jeong, H. E., Kwak, R., Khademhosseini, A. & Suh, K. Y. UV-assisted capillary force lithography for engineering biomimetic multiscale hierarchical structures: from lotus leaf to gecko foot hairs. Nanoscale 1, 331–338 (2009).
Ananthanarayanan, B., Fosbrink, M., Rahdar, M. & Zhang, J. Live-cell molecular analysis of Akt activation reveals roles for activation loop phosphorylation. J. Biol. Chem. 282, 36634–36641 (2007).
Weiger, M. C. et al. Spontaneous phosphoinositide 3-kinase signaling dynamics drive spreading and random migration of fibroblasts. J. Cell Sci. 122, 313–323 (2009).
Acknowledgements
We thank R. Alani (Boston University) for sharing melanoma cell lines and J. Zhang for sharing plasmids (UCSD); and A. Pellowe and A. L. Gonzalez (Yale University) for help with SEM imaging. J.P. is a recipient of a Samsung scholarship. This work was also supported by NIH grants U01CA15578 and CA16359 (Yale Cancer Center) to A.L. and HL107361, U54 CA141868 and P50 CA103175 to S.S.A. D.-H.K. thanks the Department of Bioengineering at the University of Washington for the new faculty startup fund.
Author information
Authors and Affiliations
Contributions
J.P., D.-H.K. and A.L. conceived and designed the project. J.P. performed the experiments. D.-H.K., H.-N.K. and M.K.K. designed and fabricated substrata under the supervision of A.L. and K.-Y.S. J.P. and E.H. tracked and analysed migration time-lapse data. C.J.W. transfected plasmids and made cell lines. A.L. and S.S.A. supervised the project. J.P. and A.L. wrote the manuscript. A.L. and S.S.A. reviewed and revised the manuscript.
Corresponding authors
Ethics declarations
Competing interests
C.J.W.’s contribution to this work occurred while she was employed by the Johns Hopkins University and does not reflect the views or policies of her current employer, the US Food and Drug Administration.
Supplementary information
Supplementary Information
Supplementary Information (PDF 13891 kb)
Supplementary Information 1
Supplementary Information 1 (TXT 0 kb)
Supplementary Information 2
Supplementary Information 2 (TXT 0 kb)
Rights and permissions
About this article
Cite this article
Park, J., Kim, DH., Kim, HN. et al. Directed migration of cancer cells guided by the graded texture of the underlying matrix. Nature Mater 15, 792–801 (2016). https://doi.org/10.1038/nmat4586
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmat4586
This article is cited by
-
Effects of nanopillars and surface coating on dynamic traction force
Microsystems & Nanoengineering (2023)
-
Retinoic acid receptor β modulates mechanosensing and invasion in pancreatic cancer cells via myosin light chain 2
Oncogenesis (2023)
-
Fabrication of micro-nano patterned materials mimicking the topological structure of extracellular matrix for biomedical applications
Nano Research (2023)
-
The glycocalyx affects the mechanotransductive perception of the topographical microenvironment
Journal of Nanobiotechnology (2022)
-
A guide to the organ-on-a-chip
Nature Reviews Methods Primers (2022)