Abstract
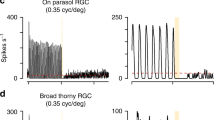
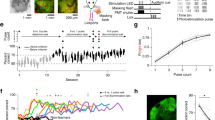
The range of natural inputs encoded by a neuron often exceeds its dynamic range. To overcome this limitation, neural populations divide their inputs among different cell classes, as with rod and cone photoreceptors, and adapt by shifting their dynamic range. We report that the dynamic behavior of retinal ganglion cells in salamanders, mice and rabbits is divided into two opposing forms of short-term plasticity in different cell classes. One population of cells exhibited sensitization—a persistent elevated sensitivity following a strong stimulus. This newly observed dynamic behavior compensates for the information loss caused by the known process of adaptation occurring in a separate cell population. The two populations divide the dynamic range of inputs, with sensitizing cells encoding weak signals and adapting cells encoding strong signals. In the two populations, the linear, threshold and adaptive properties are linked to preserve responsiveness when stimulus statistics change, with one population maintaining the ability to respond when the other fails.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Laughlin, S. A simple coding procedure enhances a neuron's information capacity. Z. Naturforsch. C. 36, 910–912 (1981).
DeWeese, M. & Zador, A. Asymmetric dynamics in optimal variance adaptation. Neural Comput. 10, 1179–1202 (1998).
Wark, B., Fairhall, A. & Rieke, F. Timescales of inference in visual adaptation. Neuron 61, 750–761 (2009).
Fairhall, A.L., Lewen, G.D., Bialek, W. & de Ruyter van Steveninck, R.R. Efficiency and ambiguity in an adaptive neural code. Nature 412, 787–792 (2001).
Nagel, K.I. & Doupe, A.J. Temporal processing and adaptation in the songbird auditory forebrain. Neuron 51, 845–859 (2006).
Maravall, M., Petersen, R.S., Fairhall, A.L., Arabzadeh, E. & Diamond, M.E. Shifts in coding properties and maintenance of information transmission during adaptation in barrel cortex. PLoS Biol. 5, e19 (2007).
Smirnakis, S.M., Berry, M.J., Warland, D.K., Bialek, W. & Meister, M. Adaptation of retinal processing to image contrast and spatial scale. Nature 386, 69–73 (1997).
Frazor, R.A. & Geisler, W.S. Local luminance and contrast in natural images. Vision Res. 46, 1585–1598 (2006).
Baccus, S.A. & Meister, M. Fast and slow contrast adaptation in retinal circuitry. Neuron 36, 909–919 (2002).
Rieke, F. & Rudd, M.E. The challenges natural images pose for visual adaptation. Neuron 64, 605–616 (2009).
Solomon, S.G., Peirce, J.W., Dhruv, N.T. & Lennie, P. Profound contrast adaptation early in the visual pathway. Neuron 42, 155–162 (2004).
Snippe, H.P. & van Hateren, J.H. Recovery from contrast adaptation matches ideal-observer predictions. J. Opt. Soc. Am. A Opt. Image Sci. Vis. 20, 1321–1330 (2003).
Brown, S.P. & Masland, R.H. Spatial scale and cellular substrate of contrast adaptation by retinal ganglion cells. Nat. Neurosci. 4, 44–51 (2001).
Pinsker, H.M., Hening, W.A., Carew, T.J. & Kandel, E.R. Long-term sensitization of a defensive withdrawal reflex in Aplysia. Science 182, 1039–1042 (1973).
Segev, R., Puchalla, J. & Berry, M.J. Functional organization of ganglion cells in the salamander retina. J. Neurophysiol. 95, 2277–2292 (2006).
Huberman, A.D. et al. Architecture and activity-mediated refinement of axonal projections from a mosaic of genetically identified retinal ganglion cells. Neuron 59, 425–438 (2008).
Dayan, P. & Abbott, L.F. Theoretical Neuroscience: Computational and Mathematical Modeling of Neural Systems 460 (MIT Press, 2009).
Abbott, L.F. & Dayan, P. The effect of correlated variability on the accuracy of a population code. Neural Comput. 11, 91–101 (1999).
Enroth-Cugell, C. & Robson, J.G. The contrast sensitivity of retinal ganglion cells of the cat. J. Physiol. (Lond.) 187, 517–552 (1966).
Srinivasan, M.V., Laughlin, S.B. & Dubs, A.T. Predictive coding: a fresh view of inhibition in the retina. Proc. R. Soc. Lond. B Biol. Sci. 216, 427–459 (1982).
Chander, D. & Chichilnisky, E.J. Adaptation to temporal contrast in primate and salamander retina. J. Neurosci. 21, 9904–9916 (2001).
Mennerick, S. & Matthews, G. Ultrafast exocytosis elicited by calcium current in synaptic terminals of retinal bipolar neurons. Neuron 17, 1241–1249 (1996).
Field, G.D. & Rieke, F. Nonlinear signal transfer from mouse rods to bipolar cells and implications for visual sensitivity. Neuron 34, 773–785 (2002).
Reinagel, P. & Reid, R.C. Temporal coding of visual information in the thalamus. J. Neurosci. 20, 5392–5400 (2000).
Wessel, R., Koch, C. & Gabbiani, F. Coding of time-varying electric field amplitude modulations in a wave-type electric fish. J. Neurophysiol. 75, 2280–2293 (1996).
Butts, D.A. How much information is associated with a particular stimulus? Network 14, 177–187 (2003).
Kim, K.J. & Rieke, F. Slow Na+ inactivation and variance adaptation in salamander retinal ganglion cells. J. Neurosci. 23, 1506–1516 (2003).
Manookin, M.B. & Demb, J.B. Presynaptic mechanism for slow contrast adaptation in mammalian retinal ganglion cells. Neuron 50, 453–464 (2006).
Rieke, F. Temporal contrast adaptation in salamander bipolar cells. J. Neurosci. 21, 9445–9454 (2001).
Brainard, D.H. The psychophysics toolbox. Spat. Vis. 10, 433–436 (1997).
Pello, D.G. The VideoToolbox software for visual psychophysics: transforming numbers into movies. Spat. Vis. 10, 437–442 (1997).
Chichilnisky, E.J. A simple white noise analysis of neuronal light responses. Network 12, 199–213 (2001).
Olveczky, B.P., Baccus, S.A. & Meister, M. Segregation of object and background motion in the retina. Nature 423, 401–408 (2003).
Gollisch, T. & Meister, M. Rapid neural coding in the retina with relative spike latencies. Science 319, 1108–1111 (2008).
Strong, S., Koberle, R. & de Ruyter van Steveninck, R. Entropy and information in neural spike trains. Phys. Rev. Lett. 80, 197–200 (1998).
Baccus, S.A. Timing and computation in inner retinal circuitry. Annu. Rev. Physiol. 69, 271–290 (2007).
Maguire, G., Maple, B., Lukasiewicz, P. & Werblin, F. Gamma-aminobutyrate type B receptor modulation of L-type calcium channel current at bipolar cell terminals in the retina of the tiger salamander. Proc. Natl. Acad. Sci. USA 86, 10144–10147 (1989).
Li, G.-L., Vigh, J. & von Gersdorff, H. Short-term depression at the reciprocal synapses between a retinal bipolar cell terminal and amacrine cells. J. Neurosci. 27, 7377–7385 (2007).
Acknowledgements
We thank E. Knudsen and W.T. Newsome for comments on the manuscript; D. Baylor, R.W. Tsien, B. Wandell, P. Jadzinsky, A.L. Fairhall, F. Rieke, D.S. Fisher and K.D. Miller for discussions; and Y. Ozuysal and A. Huberman for technical assistance. This work was supported by grants from the US National Eye Institute, Pew Charitable Trusts, McKnight Endowment Fund for Neuroscience, Karl Kirchgessner Foundation and Alfred P. Sloan Foundation (S.A.B.); and by the Stanford Medical Scientist Training Program and a US National Science Foundation Integrative Graduate Education and Research Traineeship (D.B.K.). Rabbit experiments were performed in the laboratory of M. Meister at Harvard University.
Author information
Authors and Affiliations
Contributions
D.B.K. and S.A.B. designed the study, D.B.K. performed the experiments and analysis, and D.B.K. and S.A.B. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–10 (PDF 1230 kb)
Rights and permissions
About this article
Cite this article
Kastner, D., Baccus, S. Coordinated dynamic encoding in the retina using opposing forms of plasticity. Nat Neurosci 14, 1317–1322 (2011). https://doi.org/10.1038/nn.2906
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.2906
This article is cited by
-
GABA receptors mediate adaptation and sensitization processes in mouse retinal ganglion cells
Cognitive Neurodynamics (2023)
-
Opposite forms of adaptation in mouse visual cortex are controlled by distinct inhibitory microcircuits
Nature Communications (2022)
-
Diurnal changes in the efficiency of information transmission at a sensory synapse
Nature Communications (2022)
-
Efficient and adaptive sensory codes
Nature Neuroscience (2021)
-
Functional-pathway-dominant contrast adaptation and sensitization in mouse retinal ganglion cells
Cognitive Neurodynamics (2020)