Abstract
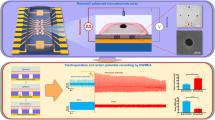
Action potentials have a central role in the nervous system and in many cellular processes, notably those involving ion channels. The accurate measurement of action potentials requires efficient coupling between the cell membrane and the measuring electrodes. Intracellular recording methods such as patch clamping involve measuring the voltage or current across the cell membrane by accessing the cell interior with an electrode, allowing both the amplitude and shape of the action potentials to be recorded faithfully with high signal-to-noise ratios1. However, the invasive nature of intracellular methods usually limits the recording time to a few hours1, and their complexity makes it difficult to simultaneously record more than a few cells. Extracellular recording methods, such as multielectrode arrays2 and multitransistor arrays3, are non-invasive and allow long-term and multiplexed measurements. However, extracellular recording sacrifices the one-to-one correspondence between the cells and electrodes, and also suffers from significantly reduced signal strength and quality. Extracellular techniques are not, therefore, able to record action potentials with the accuracy needed to explore the properties of ion channels. As a result, the pharmacological screening of ion-channel drugs is usually performed by low-throughput intracellular recording methods4. The use of nanowire transistors5,6,7, nanotube-coupled transistors8 and micro gold-spine and related electrodes9,10,11,12 can significantly improve the signal strength of recorded action potentials. Here, we show that vertical nanopillar electrodes can record both the extracellular and intracellular action potentials of cultured cardiomyocytes over a long period of time with excellent signal strength and quality. Moreover, it is possible to repeatedly switch between extracellular and intracellular recording by nanoscale electroporation and resealing processes. Furthermore, vertical nanopillar electrodes can detect subtle changes in action potentials induced by drugs that target ion channels.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Sakmann, B. & Neher, E. Single-Channel Recording, 2nd edn (Springer, 2009).
Pine, J. Recording action potentials from cultured neurons with extracellular microcircuit electrodes. J. Neurosci. Methods 2, 19–31 (1980).
Lambacher, A. et al. Electrical imaging of neuronal activity by multi-transistor-array (MTA) recording at 7.8 µm resolution. Appl.Phys. A 79, 1607–1611 (2004).
Zheng, W., Spencer, R. H. & Kiss, L. High throughput assay technologies for ion channel drug discovery. Assay Drug Dev. Technol. 2, 543–552 (2004).
Timko, B. P. et al. Electrical recording from hearts with flexible nanowire device arrays. Nano Lett. 9, 914–918 (2009).
Tian, B. et al. Three-dimensional, flexible nanoscale field-effect transistors as localized bioprobes. Science 329, 830–834 (2010).
Qing, Q. et al. Nanowire transistor arrays for mapping neural circuits in acute brain slices. Proc. Natl Acad. Sci. USA 107, 1882–1887 (2010).
Duan, X. et al. Intracellular recordings of action potentials by an extracellular nanoscale field-effect transistor. Nature Nanotech. http://doi:dx.doi.org/10.1038/nnano.2011.223 (2011).
Hai, A., Shappir, J. & Spira, M. E. In-cell recordings by extracellular microelectrodes. Nature Methods 7, 200–202 (2010).
Hai, A., Shappir, J. & Spira, M. E. Long-term, multisite, parallel, in-cell recording and stimulation by an array of extracellular microelectrodes. J. Neurophysiol. 104, 559–568 (2010).
Braeken, D. et al. Local electrical stimulation of single adherent cells using three-dimensional electrode arrays with small interelectrode distances. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2009, 2756–2759 (2009).
Choi, D. S. et al. Detection of neural signals with vertically grown single platinum nanowire-nanobud. J. Nanosci. Nanotechnol. 9, 6483–6486 (2009).
Kim, W., Ng, J. K., Kunitake, M. E., Conklin, B. R. & Yang, P. Interfacing silicon nanowires with mammalian cells. J. Am. Chem. Soc. 129, 7228–7229 (2007).
Shalek, A. K. et al. Vertical silicon nanowires as a universal platform for delivering biomolecules into living cells. Proc. Natl Acad. Sci. USA 107, 1870–1875 (2010).
Xie, C. et al. Noninvasive neuron pinning with nanopillar arrays. Nano Lett. 10, 4020–4024 (2010).
Xie, C., Hanson, L., Cui, Y. & Cui, B. Vertical nanopillars for highly localized fluorescence imaging. Proc. Natl Acad. Sci. USA 108, 3894–3899 (2011).
Claycomb, W. C. et al. HL-1 cells: a cardiac muscle cell line that contracts and retains phenotypic characteristics of the adult cardiomyocyte. Proc. Natl Acad. Sci. USA 95, 2979–2984 (1998).
Zimmermann, U., Pilwat, G. & Riemann, F. Dielectric breakdown of cell membranes. Biophys. J. 14, 881–899 (1974).
Neumann, E., Schaeferridder, M., Wang, Y. & Hofschneider, P. H. Gene-transfer into mouse lyoma cells by electroporation in high electric-fields. EMBO J. 1, 841–845 (1982).
Chang, D. C. & Reese, T. S. Changes in membrane structure induced by electroporation as revealed by rapid-freezing electron microscopy. Biophys. J. 58, 1–12 (1990).
Sartiani, L., Bochet, P., Cerbai, E., Mugelli, A. & Fischmeister, R. Functional expression of the hyperpolarization-activated, non-selective cation current I(f) in immortalized HL-1 cardiomyocytes. J. Physiol. 545, 81–92 (2002).
Tovar, O. & Tung, L. Electroporation and recovery of cardiac cell membrane with rectangular voltage pulses. Am. J. Physiol. 263, H1128–H1136 (1992).
Zipes, D. P. & Jalife, J. Cardiac Electrophysiology : From Cell to Bedside, 4th edn (Saunders, 2004).
Catterall, W. A. Structure and function of voltage-sensitive ion channels. Science 242, 50–61 (1988).
Choi, K. L., Aldrich, R. W. & Yellen, G. Tetraethylammonium blockade distinguishes two inactivation mechanisms in voltage-activated K+ channels. Proc. Natl Acad. Sci USA 88, 5092–5095 (1991).
Acknowledgements
The HL-1 cardiac cell line was obtained from William C. Claycomb (Louisiana State University). This work was supported by the NSF (CAREER award no. 1055112), the NIH (grant no. NS057906), a Searle Scholar award, a Packard Science and Engineering Fellowship (to B.C.) and a National Defense Science and Engineering Graduate Fellowship (to Z.L.)
Author information
Authors and Affiliations
Contributions
All authors conceived the experiments. C.X., Z.L. and L.H. carried out experiments. All authors contributed to the scientific planning, discussions and writing of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 632 kb)
Supplementary information
Supplementary movie (AVI 3986 kb)
Rights and permissions
About this article
Cite this article
Xie, C., Lin, Z., Hanson, L. et al. Intracellular recording of action potentials by nanopillar electroporation. Nature Nanotech 7, 185–190 (2012). https://doi.org/10.1038/nnano.2012.8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nnano.2012.8
This article is cited by
-
Graphene-integrated mesh electronics with converged multifunctionality for tracking multimodal excitation-contraction dynamics in cardiac microtissues
Nature Communications (2024)
-
Sensors-integrated organ-on-a-chip for biomedical applications
Nano Research (2023)
-
A Review of Nano/Micro/Milli Needles Fabrications for Biomedical Engineering
Chinese Journal of Mechanical Engineering (2022)
-
Nanocrown electrodes for parallel and robust intracellular recording of cardiomyocytes
Nature Communications (2022)
-
Biointerface design for vertical nanoprobes
Nature Reviews Materials (2022)