Abstract
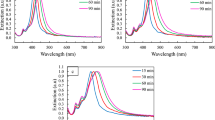
This protocol describes a method for the synthesis of Ag nanocubes and their subsequent conversion into Au nanocages via the galvanic replacement reaction. The Ag nanocubes are prepared by a rapid (reaction time < 15 min), sulfide-mediated polyol method in which Ag(I) is reduced to Ag(0) by ethylene glycol in the presence of poly(vinyl pyrrolidone) (PVP) and a trace amount of Na2S. When the concentration of Ag atoms reaches supersaturation, they agglomerate to form seeds that then grow into Ag nanostructures. The presence of both PVP and Na2S facilitate the formation of nanocubes. With this method, Ag nanocubes can be prepared and isolated for use within approximately 3 h. The Ag nanocubes can then serve as sacrificial templates for the preparation of Au nanocages, with a method for their preparation also described herein. The procedure for Au nanocage preparation and isolation requires approximately 5 h.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Kreibig, U. & Vollmer, M. Optical Properties of Metal Clusters (Springer, New York, 1995).
El-Sayed, M.A. Some interesting properties of metals confined in time and nanometer space of different shapes. Acc. Chem. Res. 34, 257–264 (2001).
Kreibig, U. & Genzel, L. Optical absorption of small metallic particles. Surf. Sci. 156, 678–700 (1987).
Elghanian, R., Storhoff, J.J., Mucic, R.C., Letsinger, R.L. & Mirkin, C.A. Selective colormetric detection of polynucleotides based on the distance-dependent optical properties of gold nanoparticles. Science 277, 1078–1081 (1997).
West, J.L. & Halas, N.J. Engineered nanoparticles for biophotonics applications: improving sensing, imaging, and therapeutics. Annu. Rev. Biomed. Eng. 5, 285–292 (2003).
Chen, J. et al. Gold nanocages: engineering the structure for biomedical applications. Adv. Mater. Weinheim. 17, 2255–2261 (2005).
Mie, G. Beitrage zur Optik truber Medien, speziell kolloidaler Metallosungen. Ann. Phys. 25, 377–445 (1908).
Hu, M. et al. Gold nanostructures: engineering their plasmonic properties for biomedical applications. Chem. Soc. Rev. 35, 1084–1094 (2006).
Sun, Y. & Xia, Y. Mechanistic study on the replacement reaction between silver nanostructures and chloroauric acid in the aqueous medium. J. Am. Chem. Soc. 126, 3892–3901 (2004).
Chen, J. et al. Gold nanocages: bioconjugation and their potential use as optical imaging contrast agents. Nano Lett. 5, 473–477 (2005).
Cang, H. et al. Gold nanocages as contrast agents for spectroscopic optical coherence tomography. Opt. Lett. 30, 3048–3050 (2005).
Chen, J. et al. Immuno gold nanocages with tailored optical properties for targeted photothermal destruction of cancer cells. Nano Lett. 7, 1318–1322 (2007).
Sun, Y. & Xia, Y. Shape-controlled synthesis of gold and silver nanoparticles. Science 298, 2176–2179 (2002).
Wiley, B., Herricks, T., Sun, Y. & Xia, Y. Polyol synthesis of silver nanoparticles: use of chloride and oxygen to promote the formation of single-crystal, truncated cubes and tetrahedrons. Nano Lett. 4, 1733–1739 (2004).
Im, S.H., Lee, Y.T., Wiley, B. & Xia, Y. Large-scale synthesis of silver nanocubes: the role of HCl in promoting cube perfection and monodispersity. Angew. Chem. Int. Ed. Engl. 44, 2154–2157 (2005).
Wiley, B., Sun, Y. & Xia, Y. Polyol synthesis of silver nanostructures: control of product morphology with Fe(II) or Fe(III) species. Langmuir 21, 8077–8080 (2005).
Siekkinen, A.R., McLellan, J.M., Chen, J. & Xia, Y. Rapid synthesis of small silver nanocubes by mediating polyol reduction with a trace amount of sodium sulfide or sodium hydrosulfide. Chem. Phys. Lett. 432, 491–496 (2006).
Wiley, B. et al. Maneuvering the surface plasmon resonance of silver nanostructures through shape-controlled synthesis. J. Phys. Chem. B Condens. Matter Mater. Surf. Interfaces Biophys. 110, 15666–15675 (2006).
Sun, Y., Mayers, B. & Xia, Y. Template-engaged replacement reaction: a one-step approach to the large-scale synthesis of metal nanostructures with hollow interiors. Nano Lett. 2, 481–485 (2002).
Sun, Y., Mayers, B. & Xia, Y. Metal nanostructures with hollow interiors. Adv. Mater. Weinheim 15, 641–646 (2003).
Sun, Y. & Xia, Y. Triangular nanoplates of silver: synthesis, characterization, and their use as sacrificial templates in generating triangular nanorings of gold. Adv. Mater. Weinheim 15, 695–699 (2003).
Acknowledgements
This work was supported in part by a Director's Pioneer Award (5DP1OD000798 to Y.X.) from National Institutes of Health (NIH), a grant from National Science Foundation (NSF) (DMR-0451788 to Y.X.), a grant from NIH (5R01CA120480 to X.L.), a DARPA-DURINT subcontract from Harvard University, and a fellowship from David and Lucile Packard Foundation. Y.X. is an Alfred P. Sloan Research Fellow (2000–2005) and a Camille Dreyfus Teacher Scholar (2002–2007). L.A. thanks the UW Center for Nanotechnology for an IGERT Student Fellowship jointly sponsored by NSF and National Cancer Institute (NCI). Part of the work was performed at the UW Nanotech User Facility (NTUF), a member of the National Nanotechnology Infrastructure Network (NNIN) funded by NSF.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Skrabalak, S., Au, L., Li, X. et al. Facile synthesis of Ag nanocubes and Au nanocages. Nat Protoc 2, 2182–2190 (2007). https://doi.org/10.1038/nprot.2007.326
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2007.326
This article is cited by
-
Near-infrared-featured broadband CO2 reduction with water to hydrocarbons by surface plasmon
Nature Communications (2023)
-
Hyperthermia-triggered biomimetic bubble nanomachines
Nature Communications (2023)
-
Implantable versatile oxidized bacterial cellulose membrane for postoperative HNSCC treatment via photothermal-boosted immunotherapy
Nano Research (2023)
-
Cascaded hot electron transfer within plasmonic Ag@Pt heterostructure for enhanced electrochemical reactions
Science China Materials (2023)
-
Extracellular matrix modulating enzyme functionalized biomimetic Au nanoplatform-mediated enhanced tumor penetration and synergistic antitumor therapy for pancreatic cancer
Journal of Nanobiotechnology (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.