Abstract
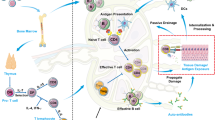
Type 1 diabetes mellitus (T1DM) is an autoimmune disorder directed against the β cells of the pancreatic islets. The genetic risk of the disease is linked to HLA-DQ risk alleles and unknown environmental triggers. In most countries, only 10–15% of children or young adults newly diagnosed with T1DM have a first-degree relative with the disease. Autoantibodies against insulin, GAD65, IA-2 or the ZnT8 transporter mark islet autoimmunity. These islet autoantibodies may already have developed in children of 1–3 years of age. Immune therapy in T1DM is approached at three different stages. Primary prevention is treatment of individuals at increased genetic risk. For example, one trial is testing if hydrolyzed casein milk formula reduces T1DM incidence in genetically predisposed infants. Secondary prevention is targeted at individuals with persistent islet autoantibodies. Ongoing trials involve nonautoantigen-specific therapies, such as Bacillus Calmette–Guérin vaccine or anti-CD3 monoclonal antibodies, or autoantigen-specific therapies, including oral and nasal insulin or alum-formulated recombinant human GAD65. Trial interventions at onset of T1DM have also included nonautoantigen-specific approaches, and autoantigen-specific therapies, such as proinsulin peptides. Although long-term preservation of β-cell function has been difficult to achieve in many studies, considerable progress is being made through controlled clinical trials and animal investigations towards uncovering mechanisms of β-cell destruction. Novel therapies that prevent islet autoimmunity or halt progressive β-cell destruction are needed.
Key Points
-
As an autoimmune disease, type 1 diabetes mellitus (T1DM) is strongly associated with mutations in HLA-DQ risk alleles; non-HLA genes contributing to disease risk are related to the immune system
-
Autoantibodies to the β-cell autoantigens insulin, GAD65, IA-2 and the ZnT8 transporter are major markers of islet autoimmunity; the number of islet autoantibodies determines risk and time to T1DM clinical onset
-
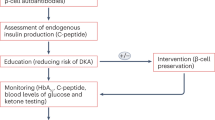
Immune therapy for T1DM is approached at three stages: primary prevention, secondary prevention and intervention
-
Primary prevention requires identification of newborn babies at increased genetic risk of T1DM; induction of immunological tolerance to islet autoantigens is a goal, but is difficult to measure
-
Nonautoantigen-specific or autoantigen-specific interventions can be used at the secondary prevention stage in individuals who have developed persistent islet autoantibodies; combination therapies have yet to be carried out
-
Immune therapy interventions at onset of T1DM can involve both nonautoantigen-specific and autoantigen-specific therapies; the most common primary outcome in clinical studies and trials is the preservation of C-peptide levels
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Hirsch, I. B. Insulin analogues. N. Engl. J. Med. 352, 174–183 (2005).
Steenkiste, A. et al. 14th International HLA and Immunogenetics Workshop: report on the HLA component of type 1 diabetes. Tissue Antigens 69 (Suppl. 1), 214–225 (2007).
Concannon, P., Rich, S. S. & Nepom, G. T. Genetics of type 1A diabetes. N. Engl. J. Med. 360, 1646–1654 (2009).
La Torre, D. & Lernmark, A. Immunology of β-cell destruction. Adv. Exp. Med. Biol. 654, 537–583 (2010).
Pihoker, C., Gilliam, L. K., Hampe, C. S. & Lernmark, A. Autoantibodies in diabetes. Diabetes 54 (Suppl. 2), 52–61 (2005).
Lernmark, A. et al. The Fourth International Serum Exchange Workshop to standardize cytoplasmic islet cell antibodies. The Immunology and Diabetes Workshops and Participating Laboratories. Diabetologia 34, 534–535 (1991).
Törn, C. et al. Diabetes Antibody Standardization Program: evaluation of assays for autoantibodies to glutamic acid decarboxylase and islet antigen-2. Diabetologia 51, 846–852 (2008).
Bonifacio, E. et al. Harmonization of glutamic acid decarboxylase and islet antigen-2 autoantibody assays for national institute of diabetes and digestive and kidney diseases consortia. J. Clin. Endocrinol. Metab. 95, 3360–3367 (2010).
Mallone, R., Brezar, V. & Boitard, C. T cell recognition of autoantigens in human type 1 diabetes: clinical perspectives. Clin. Dev. Immunol. 2011, 513210 (2011).
Orban, T. et al. Pancreatic islet autoantibodies as predictors of type 1 diabetes in the Diabetes Prevention Trial-Type 1. Diabetes Care 32, 2269–2274 (2009).
Barker, J. M. et al. Prediction of autoantibody positivity and progression to type 1 diabetes: Diabetes Autoimmunity Study in the Young (DAISY). J. Clin. Endocrinol. Metab. 89, 3896–3902 (2004).
Elding Larsson, H. et al. Reduced prevalence of diabetic ketoacidosis at diagnosis of type 1 diabetes in young children participating in longitudinal follow-up. Diabetes Care 34, 2347–2352 (2011).
Wallensteen, M. et al. Factors influencing the magnitude, duration, and rate of fall of B-cell function in type 1 (insulin-dependent) diabetic children followed for two years from their clinical diagnosis. Diabetologia 31, 664–669 (1988).
Bach, J. F. & Chatenoud, L. A historical view from thirty eventful years of immunotherapy in autoimmune diabetes. Semin. Immunol. 23, 174–181 (2011).
Greenbaum, C. J., Schatz, D. A., Haller, M. J. & Sanda, S. Through the fog: recent clinical trials to preserve beta-cell function in type 1 diabetes. Diabetes 61, 1323–1330 (2012).
Knip, M. et al. Early feeding and risk of type 1 diabetes: experiences from the Trial to Reduce Insulin-dependent diabetes mellitus in the Genetically at Risk (TRIGR). Am. J. Clin. Nutr. 94 (Suppl. 6), 1814–1820 (2011).
Knip, M. et al. Dietary intervention in infancy and later signs of β-cell autoimmunity. N. Engl. J. Med. 363, 1900–1908 (2010).
Wicklow, B. A. & Taback, S. P. Feasibility of a type 1 diabetes primary prevention trial using 2000 IU vitamin D3 in infants from the general population with increased HLA-associated risk. Ann. NY Acad. Sci. 1079, 310–312 (2006).
Bock, G. et al. The effect of vitamin D supplementation on peripheral regulatory T cells and β cell function in healthy humans: a randomized controlled trial. Diabetes Metab. Res. Rev. 27, 942–945 (2011).
Sørensen, I. M. et al. Maternal serum levels of 25-hydroxy-vitamin D during pregnancy and risk of type 1 diabetes in the offspring. Diabetes 61, 175–178 (2012).
Stene, L. C. & Joner, G. Use of cod liver oil during the first year of life is associated with lower risk of childhood-onset type 1 diabetes: a large, population-based, case–control study. Am. J. Clin. Nutr. 78, 1128–1134 (2003).
Chase, H. P. et al. Nutritional Intervention to Prevent (NIP) Type 1 Diabetes: a pilot trial. Infant Child. Adolesc. Nutr. 1, 98–107 (2009).
Hagopian, W. A. et al. TEDDY—The Environmental Determinants of Diabetes in the Young: an observational clinical trial. Ann. NY Acad. Sci. 1079, 320–326 (2006).
Hagopian, W. A. et al. The Environmental Determinants of Diabetes in the Young (TEDDY): genetic criteria and international diabetes risk screening of 421000 infants. Pediatr. Diabetes 12, 733–743 (2011).
Achenbach, P., Barker, J. & Bonifacio, E. Modulating the natural history of type 1 diabetes in children at high genetic risk by mucosal insulin immunization. Curr. Diab. Rep. 8, 87–93 (2008).
Diabetes Prevention Trial—Type 1 Diabetes Study Group. Effects of insulin in relatives of patients with type 1 diabetes mellitus. N. Engl. J. Med. 346, 1685–1691 (2002).
Andersson, C. et al. The three ZNT8 autoantibody variants together improve the diagnostic sensitivity of childhood and adolescent type 1 diabetes. Autoimmunity 44, 394–405 (2011).
Carel, J. C., Boitard, C., Eisenbarth, G., Bach, J. F. & Bougneres, P. F. Cyclosporine delays but does not prevent clinical onset in glucose intolerant pre-type 1 diabetic children. J. Autoimmun. 9, 739–745 (1996).
Huppmann, M., Baumgarten, A., Ziegler, A. G. & Bonifacio, E. Neonatal Bacille Calmette-Guerin vaccination and type 1 diabetes. Diabetes Care 28, 1204–1206 (2005).
Bohmer, K. P. et al. Linear loss of insulin secretory capacity during the last six months preceding IDDM. No effect of antiedematous therapy with ketotifen. Diabetes Care 17, 138–141 (1994).
Gale, E. A., Bingley, P. J., Emmett, C. L. & Collier, T. European Nicotinamide Diabetes Intervention Trial (ENDIT): a randomised controlled trial of intervention before the onset of type 1 diabetes. Lancet 363, 925–931 (2004).
Lampeter, E. F. et al. The Deutsche Nicotinamide Intervention Study: an attempt to prevent type 1 diabetes. DENIS Group. Diabetes 47, 980–984 (1998).
Ziegler, A. G., Schmid, S., Huber, D., Hummel, M. & Bonifacio, E. Early infant feeding and risk of developing type 1 diabetes-associated autoantibodies. JAMA 290, 1721–1728 (2003).
Norris, J. M. et al. Timing of initial cereal exposure in infancy and risk of islet autoimmunity. JAMA 290, 1713–1720 (2003).
Hummel, S., Pfülger, M., Hummel, M., Bonifacio, E. & Ziegler, A. G. Primary dietary intervention study to reduce the risk of islet autoimmunity in children at increased risk for type 1 diabetes: the BABYDIET study. Diabetes Care 34, 1301–1305 (2011).
Keller, R. J., Eisenbarth, G. S. & Jackson, R. A. Insulin prophylaxis in individuals at high risk of type I diabetes. Lancet 341, 927–928 (1993).
Pugliese, A. et al. HLA-DQB1*0602 is associated with dominant protection from diabetes even among islet cell antibody-positive first-degree relatives of patients with IDDM. Diabetes 44, 608–613 (1995).
Skyler, J. S. et al. Effects of oral insulin in relatives of patients with type 1 diabetes: The Diabetes Prevention Trial--Type 1. Diabetes Care 28, 1068–1076 (2005).
Vehik, K. et al. Long-term outcome of individuals treated with oral insulin: diabetes prevention trial-type 1 (DPT-1) oral insulin trial. Diabetes Care 34, 1585–1590 (2011).
Harrison, L. C. et al. Pancreatic beta-cell function and immune responses to insulin after administration of intranasal insulin to humans at risk for type 1 diabetes. Diabetes Care 27, 2348–2355 (2004).
Näntö-Salonen, K. et al. Nasal insulin to prevent type 1 diabetes in children with HLA genotypes and autoantibodies conferring increased risk of disease: a double-blind, randomised controlled trial. Lancet 372, 1746–1755 (2008).
Ryhänen, S. J. et al. Impact of intranasal insulin on insulin antibody affinity and isotypes in young children with HLA-conferred susceptibility to type 1 diabetes. Diabetes Care 34, 1383–1388 (2011).
Agardh, C. D. et al. Clinical evidence for the safety of GAD65 immunomodulation in adult-onset autoimmune diabetes. J. Diabetes Complications 19, 238–246 (2005).
Agardh, C. D., Lynch, K. F., Palmer, M., Link, K. & Lernmark, A. GAD65 vaccination: 5 years of follow-up in a randomised dose-escalating study in adult-onset autoimmune diabetes. Diabetologia 52, 1363–1368 (2009).
Ludvigsson, J. et al. GAD treatment and insulin secretion in recent-onset type 1 diabetes. N. Engl. J. Med. 359, 1909–1920 (2008).
Wherrett, D. K. et al. Antigen-based therapy with glutamic acid decarboxylase (GAD) vaccine in patients with recent-onset type 1 diabetes: a randomised double-blind trial. Lancet 378, 319–327 (2011).
Leslie, R. D. G. & Pyke, D. A. in Immunology of Diabetes (ed. Irvine, J.) 345–347 (Teviot Scientific Publications, Edinburgh, 1980).
Skyler, J. S. Immune intervention studies in insulin-dependent diabetes mellitus. Diabetes Metab. Rev. 3, 1017–1035 (1987).
Ludvigsson, J., Heding, L. G., Larsson, Y. & Leander, E. C-peptide in juvenile diabetics beyond the postinitial remission period. Relation to clinical manifestations at onset of diabetes, remission and diabetic control. Acta Paediatr. Scand. 66, 177–184 (1977).
Rodriquez, H. et al. The prevention of diabetes progression trial (PDPT): preservation of beta-cell function using daclizumab in new onset type diabetes. Pediatric Diabetes 7 (Suppl. S5), 58–O3 (2006).
Waldron-Lynch, F. & Herold, K. C. Immunomodulatory therapy to preserve pancreatic beta-cell function in type 1 diabetes. Nat. Rev. Drug Discov. 10, 439–452 (2011).
Keymeulen, B. et al. Four-year metabolic outcome of a randomised controlled CD3-antibody trial in recent-onset type 1 diabetic patients depends on their age and baseline residual beta cell mass. Diabetologia 53, 614–623 (2010).
Keymeulen, B. et al. Insulin needs after CD3-antibody therapy in new-onset type 1 diabetes. N. Engl. J. Med. 352, 2598–2608 (2005).
Herold, K. C. et al. Treatment of patients with new onset type 1 diabetes with a single course of anti-CD3 mAb teplizumab preserves insulin production for up to 5 years. Clin. Immunol. 132, 166–173 (2009).
Herold, K. C. et al. Anti-CD3 monoclonal antibody in new-onset type 1 diabetes mellitus. N. Engl. J. Med. 346, 1692–1698 (2002).
Herold, K. C. et al. A single course of anti-CD3 monoclonal antibody hOKT3γ1(Ala-Ala) results in improvement in C-peptide responses and clinical parameters for at least 2 years after onset of type 1 diabetes. Diabetes 54, 1763–1769 (2005).
Larsson, H. E. & Lernmark, A. Vaccination against type 1 diabetes. J. Intern. Med. 269, 626–635 (2011).
Sherry, N. et al. Teplizumab for treatment of type 1 diabetes (Protégé study): 1-year results from a randomised, placebo-controlled trial. Lancet 378, 487–497 (2011).
Cernea, S. & Herold, K. C. Monitoring of antigen-specific CD8 T cells in patients with type 1 diabetes treated with antiCD3 monoclonal antibodies. Clin. Immunol. 134, 121–129 (2010).
Pescovitz, M. D. et al. Rituximab, B-lymphocyte depletion, and preservation of beta-cell function. N. Engl. J. Med. 361, 2143–2152 (2009).
Marino, E., Tan, B., Binge, L., Mackay, C. R. & Grey, S. T. B-cell cross-presentation of autologous antigen precipitates diabetes. Diabetes 61, 2893–2905 (2012).
Pescovitz, M. D. et al. Effect of rituximab on human in vivo antibody immune responses. J. Allergy Clin. Immunol. 128, 1295–1302.e5 (2011).
Yu, L. et al. Rituximab selectively suppresses specific islet antibodies. Diabetes 60, 2560–2565 (2011).
Sumpter, K. M., Adhikari, S., Grishman, E. K. & White, P. C. Preliminary studies related to anti-interleukin-1β therapy in children with newly diagnosed type 1 diabetes. Pediatr. Diabetes 12, 656–667 (2011).
Shehadeh, N. et al. Effect of adjuvant therapy on development of diabetes in mouse and man. Lancet 343, 706–707 (1994).
Elliott, J. F., Marlin, K. L. & Couch, R. M. Effect of bacille Calmette–Guérin vaccination on C-peptide secretion in children newly diagnosed with IDDM. Diabetes Care 21, 1691–1693 (1998).
Allen, H. F. et al. Effect of Bacillus Calmette–Guerin vaccination on new-onset type 1 diabetes. A randomized clinical study. Diabetes Care 22, 1703–1707 (1999).
Faustman, D. L. et al. Proof-of-concept, randomized, controlled clinical trial of Bacillus–Calmette–Guerin for treatment of long-term type 1 diabetes. PLoS ONE 7, e41756 (2012).
Orban, T. et al. Co-stimulation modulation with abatacept in patients with recent-onset type 1 diabetes: a randomised, double-blind, placebo-controlled trial. Lancet 378, 412–419 (2011).
Fischer, B., Elias, D., Bretzel, R. G. & Linn., T . Immunomodulation with heat shock protein DiaPep277 to preserve β cell function in type 1 diabetes—an update. Expert Opin. Biol. Ther. 10, 265–272 (2010).
Walter, M., Philotheou, A., Bonnici, F., Ziegler, A. G. & Jimenez, R. No effect of the altered peptide ligand NBI-6024 on β-cell residual function and insulin needs in new-onset type 1 diabetes. Diabetes Care 32, 2036–2040 (2009).
Garren, H. Biological Therapeutics Research and Development—GTCbio's Fifth Annual Conference. October 20–22, 2010, San Francisco, CA, USA. IDrugs 13, 840–842 (2010).
Ludvigsson, J. et al. GAD65 antigen therapy in recently diagnosed type 1 diabetes mellitus. N. Engl. J. Med. 366, 433–442 (2012).
Axelsson, S. et al. Long-lasting immune responses 4 years after GAD-alum treatment in children with type 1 diabetes. PLoS ONE 6, e29008 (2011).
Long, S. A. et al. Rapamycin/IL-2 combination therapy in patients with type 1 diabetes augments tregs yet transiently impairs β-cell function. Diabetes 61, 2340–2348 (2012).
Gottlieb, P. A. et al. Failure to preserve beta-cell function with mycophenolate mofetil and daclizumab combined therapy in patients with new-onset type 1 diabetes. Diabetes Care 33, 826–832 (2010).
Bresson, D. & von Herrath, M. Resuscitating adaptive Tregs with combination therapies? Novartis Found. Symp. 292, 50–60 (2008).
Wicker, L. S. et al. Naturally processed T cell epitopes from human glutamic acid decarboxylase identified using mice transgenic for the type 1 diabetes-associated human MHC class II allele, DRB1*0401. J. Clin. Invest. 98, 2597–2603 (1996).
Steed, J., Gilliam, L. K., Harris, R. A., Lernmark, A. & Hampe, C. S. Antigen presentation of detergent-free glutamate decarboxylase (GAD65) is affected by human serum albumin as carrier protein. J. Immunol. Methods 334, 114–121 (2008).
Thunander, M., Thorgeirsson, H., Törn, C., Petersson, C. & Landin-Olsson, M. β-cell function and metabolic control in latent autoimmune diabetes in adults with early insulin versus conventional treatment: a 3-year follow-up. Eur. J. Endocrinol. 164, 239–245 (2011).
Carlsson, A. et al. Low risk HLA-DQ and increased body mass index in newly diagnosed type 1 diabetes children in the Better Diabetes Diagnosis study in Sweden. Int. J. Obes. 36, 718–724 (2012).
Acknowledgements
The studies in the authors laboratories were supported by the National Institutes of Health (NIH grant DK063861), Juvenile Diabetes Research Foundation (grant 17-2011-576), Swedish Research Council (grant K2011-54X-15312-07-6), Swedish Childhood Diabetes Fund (H. E. Larsson), Swedish Diabetes Foundation (Å. Lernmark), Skåne County Council for Research and Development, SUS Fund, and DIAPREPP (EU grant 202013).
Author information
Authors and Affiliations
Contributions
Both authors researched data for the article, provided a substantial contribution to discussion of content, wrote the article and reviewed and/or edited the article before submission.
Corresponding author
Ethics declarations
Competing interests
Å. Lernmark declares associations with the following companies: Diamyd Medical AB (consultant, grant/research support, patent holder/applicant), Probi AB (consultant) and Zealand Pharma (consultant). H. E. Larsson declares no competing interests.
Rights and permissions
About this article
Cite this article
Lernmark, Å., Larsson, H. Immune therapy in type 1 diabetes mellitus. Nat Rev Endocrinol 9, 92–103 (2013). https://doi.org/10.1038/nrendo.2012.237
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrendo.2012.237
This article is cited by
-
Metabolic Messengers: glucagon
Nature Metabolism (2023)
-
Stem cells differentiation into insulin-producing cells (IPCs): recent advances and current challenges
Stem Cell Research & Therapy (2022)
-
Pancreas regeneration
Nature (2018)
-
Targeted delivery of antigen to intestinal dendritic cells induces oral tolerance and prevents autoimmune diabetes in NOD mice
Diabetologia (2018)
-
Role of TGF-β in Self-Peptide Regulation of Autoimmunity
Archivum Immunologiae et Therapiae Experimentalis (2018)