Key Points
-
To mediate an effective immune response, lymphocytes must find their way to secondary lymphoid tissues, such as the lymph nodes, the Peyer's patches and the spleen, where antigens and antigen-presenting dendritic cells are selectively localized.
-
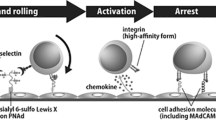
When entering lymph nodes and Peyer's patches, lymphocytes selectively adhere to and transmigrate through the high endothelial venules (HEVs), which are distinct from normal venules in several ways.
-
HEVs express unique adhesion molecules (vascular addressins) that function as ligands for lymphocyte homing receptors. They also express several lymphoid chemokines that can activate integrins on circulating lymphocytes.
-
Accumulating evidence indicates that chemokines produced in and around HEVs have a crucial role in lymphocyte trafficking to lymph nodes and Peyer's patches. Nevertheless, the way that chemokines function in vivo is not fully understood.
-
Numerous chemokine-binding molecules are expressed in and immediately around HEVs. Certain chemokines bind preferentially to certain chemokine-binding molecules that seem to be expressed in a concentric manner in the HEV area.
-
We propose that each chemokine-binding molecule provides a substrate to present the appropriate bound chemokine and that lymphocytes expressing appropriate chemokine receptors respond to these matrix-presented chemokines successively. This would result in directed trafficking of lymphocytes across HEVs and into the lymph-node parenchyma.
-
Lymphocytes might respond to these chemokines by haptotaxis and/or chemokinesis, rather than by chemotaxis.
-
Numerous questions remain unanswered concerning the physiology and function of HEVs.
Abstract
Lymphocytes are intrinsically mobile and circulate continuously between the blood and secondary lymphoid tissues. When naive lymphocytes first enter lymph nodes and Peyer's patches, they adhere to and migrate across specific blood vessels known as high endothelial venules (HEVs). The local availability of chemokines in or near HEVs is crucial for the specificity of this process. Here, we summarize recent studies of the chemokine-directed events in lymphocyte trafficking across HEVs, and we examine the dogmas and enigmas concerning lymphocyte migration to lymph nodes and Peyer's patches. A model is also discussed, in which we propose that the response to chemokines immobilized on extracellular-matrix components is important for lymphocyte positioning in vivo.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Butcher, E. C. The regulation of lymphocyte traffic. Curr. Top. Microbiol. Immunol. 128, 85–122 (1986).
Kraal, G. & Mebius, R. E. High endothelial venules: lymphocyte traffic control and controlled traffic. Adv. Immunol. 65, 347–395 (1997).
Gowans, J. L. in Adhesion Molecules and Chemokines in Lymphocyte Trafficking (ed. Hamann, A.) ix–xi (Harwood Academic Publishers, Amsterdam, 1997).
Westermann, J. & Pabst, R. How organ-specific is the migration of 'naïve' and 'memory' T cells? Immunol. Today 17, 278–282 (1996).
Pabst, R. & Westermann, J. in Adhesion Molecules and Chemokines in Lymphocyte Trafficking (ed. Hamann, A.) 21–37 (Harwood Academic Publishers, Amsterdam, 1997).
Yoffey, J. M. & Courtice, F. C. in Lymphatics, Lymph and the Lymphomyeloid Complex (eds Yoffey, J. M. & Courtice, F. C.) 539–551 (Academic Press, London, 1970).
Smith, J. B., McIntosh, G. H. & Morris, B. The traffic of cells through tissues: a study of peripheral lymph in sheep. J. Anat. 107, 87–100 (1970).
Hein, W. R., McClure, S. J. & Miyasaka, M. Cellular composition of peripheral lymph and skin of sheep defined by monoclonal antibodies. Int. Arch. Allergy Appl. Immunol. 84, 241–246 (1987).
Cella, M. et al. Plasmacytoid monocytes migrate to inflamed lymph nodes and produce large amounts of type I interferon. Nature Med. 5, 919–923 (1999).
Banchereau, J. et al. Immunobiology of dendritic cells. Annu. Rev. Immunol. 18, 767–811 (2000).
Cavanagh, L. L. & von Andrian, U. Travellers in many guises: the origins and destinations of dendritic cells. Immunol. Cell Biol. 80, 448–462 (2002).
Gretz, J. E., Anderson, A. O. & Shaw, S. Cords, channels, corridors and conduits: critical architectural elements facilitating cell interactions in the lymph node cortex. Immunol. Rev. 156, 11–24 (1997). This paper, together with references 58 and 59, describes a special system for delivering small molecules from the subcapsular sinus to the HEVs in lymph nodes.
Bajenoff, M., Granjeaud, S. & Guerder, S. The strategy of T cell antigen-presenting cell encounter in antigen-draining lymph nodes revealed by imaging of initial T cell activation. J. Exp. Med. 198, 715–724 (2003).
Hay, J. B. & Hobbs, B. B. The flow of blood to lymph nodes and its relation to lymphocyte traffic and the immune response. J. Exp. Med. 145, 31–44 (1977).
Butcher, E. C. et al. Lymphocyte trafficking and regional immunity. Adv. Immunol. 72, 209–253 (1999).
Homeister, J. W. et al. The α(1,3)fucosyltransferases FucT-IV and FucT-VII exert collaborative control over selectin-dependent leukocyte recruitment and lymphocyte homing. Immunity 15, 115–126 (2001).
Maly, P. et al. The α(1,3)fucosyltransferase Fuc-TVII controls leukocyte trafficking through an essential role in L-, E-, and P-selectin ligand biosynthesis. Cell 86, 643–653 (1996).
Hemmerich, S. et al. Sulfation of L-selectin ligands by an HEV-restricted sulfotransferase regulates lymphocyte homing to lymph nodes. Immunity 15, 237–247 (2001).
Hiraoka, N. et al. A novel, high endothelial venule-specific sulfotransferase expresses 6-sulfo sialyl Lewisx, an L-selectin ligand displayed by CD34. Immunity 11, 79–89 (1999).
Warnock, R. A. et al. Molecular mechanisms of lymphocyte homing to peripheral lymph nodes. J. Exp. Med. 187, 205–216 (1998).
von Andrian, U. H. & M'Rini, C. In situ analysis of lymphocyte migration to lymph nodes. Cell Adhes. Commun. 6, 85–96 (1998).
Dustin, M. L. & Springer, T. A. T-cell receptor cross-linking transiently stimulates adhesiveness through LFA-1. Nature 341, 619–624 (1989).
Spangrude, G. J., Baaten, B. A. & Daynes, R. A. Molecular mechanisms of lymphocyte extravasation. I. Studies of two selective inhibitors of lymphocyte recirculation. J. Immunol. 132, 354–362 (1984).
Bargatze, R. F. & Butcher, E. C. Rapid G protein-regulated activation event involved in lymphocyte binding to high endothelial venules. J. Exp. Med. 178, 367–372 (1993).
Hamann, A. et al. Evidence for an accessory role of LFA-1 in lymphocyte-high endothelium interaction during homing. J. Immunol. 140, 693–699 (1988).
Campbell, J. J. et al. Chemokines and the arrest of lymphocyte rolling under flow conditions. Science 279, 381–384 (1998). The first evidence that chemokines can induce arrest of rolling lymphocytes under flow conditions.
Cyster, J. G. Chemokines and cell migration in secondary lymphoid organs. Science 286, 2098–2102 (1999).
Kunkel, E. J. & Butcher, E. C. Chemokines and the tissue-specific migration of lymphocytes. Immunity 16, 1–4 (2002).
Campbell, J. J. et al. 6-C-kine (SLC), a lymphocyte adhesion-triggering chemokine expressed by high endothelium, is an agonist for the MIP-3-β receptor CCR7. J. Cell Biol. 141, 1053–1059 (1998).
Yoshida, R. et al. Secondary lymphoid-tissue chemokine is a functional ligand for the CC chemokine receptor CCR7. J. Biol. Chem. 273, 7118–7122 (1998).
Nagira, M. et al. Molecular cloning of a novel human CC chemokine secondary lymphoid-tissue chemokine that is a potent chemoattractant for lymphocytes and mapped to chromosome 9p13. J. Biol. Chem. 272, 19518–19524 (1997).
Gunn, M. D. et al. A chemokine expressed in lymphoid high endothelial venules promotes the adhesion and chemotaxis of naïve T lymphocytes. Proc. Natl Acad. Sci. USA 95, 258–263 (1998).
Vassileva, G. et al. The reduced expression of 6Ckine in the plt mouse results from the deletion of one of two 6Ckine genes. J. Exp. Med. 190, 1183–1188 (1999).
Tangemann, K., Gunn, M. D., Giblin, P. & Rosen, S. D. A high-endothelial cell derived chemokine induces rapid, efficient, and subset-specific arrest of rolling T lymphocytes on a reconstituted endothelial substrate. J. Immunol. 161, 6330–6337 (1998).
Stein, J. V. et al. The CC chemokine thymus-derived chemotactic agent 4 (TCA-4, secondary lymphoid tissue chemokine, 6Ckine, exodus-2) triggers lymphocyte function-associated antigen 1-mediated arrest of rolling T lymphocytes in peripheral lymph node high endothelial venules. J. Exp. Med. 191, 61–76 (2000).
Pachynski, R. K., Wu, S. W., Gunn, M. D. & Erle, D. J. Secondary lymphoid-tissue chemokine (SLC) stimulates integrin α4β7-mediated adhesion of lymphocytes to mucosal addressin cell adhesion molecule-1 (MAdCAM-1) under flow. J. Immunol. 161, 952–956 (1998).
Ebisuno, Y. et al. Cutting edge: the B cell chemokine CXC chemokine ligand 13/B lymphocyte chemoattractant is expressed in the high endothelial venules of lymph nodes and Peyer's patches and affects B cell trafficking across high endothelial venules. J. Immunol. 171, 1642–1646 (2003). The first paper to describe that the expression of CXCL13 in the HEV lumen is important for B-cell trafficking across HEVs in both lymph nodes and Peyer's patches.
Baekkevold, E. S. et al. The CCR7 ligand ELC (CCL19) is transcytosed in high endothelial venules and mediates T cell recruitment. J. Exp. Med. 193, 1105–1111 (2001).
Luther, S. A. et al. Coexpression of the chemokine ELC and SLC by T zone stromal cells and deletion of the ELC gene in the plt/plt mouse. Proc. Natl Acad. Sci. USA 97, 12694–12699 (2000).
Nakano, H. & Gunn, M. D. Gene duplications at the chemokine locus on mouse chromosome 4: multiple strain-specific haplotypes and the deletion of secondary lymphoid-organ chemokine and EBI-1 ligand chemokine genes in the plt mutation. J. Immunol. 166, 361–369 (2001).
Nakano, H. et al. Genetic defect in T lymphocyte-specific homing into peripheral lymph nodes. Eur. J. Immunol. 27, 215–221 (1997).
Forster, R. et al. CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell 99, 23–33 (1999).
Grabovsky, V. et al. Subsecond induction of α4 integrin clustering by immobilized chemokines stimulates leukocyte tethering and rolling on endothelial vascular cell adhesion molecule 1 under flow conditions. J. Exp. Med. 192, 495–506 (2000).
Okada, T. et al. Chemokine requirements for B cell entry to lymph nodes and Peyer's patches. J. Exp. Med. 196, 65–75 (2002). This study delineates the importance of CCR7 and CXCR4 in transmitting integrin-activating signals to rolling T and B cells in HEVs.
von Andrian, U. H. & Mempel, T. R. Homing and cellular traffic in lymph nodes. Nature Rev. Immunol. 3, 867–878 (2003).
Phillips, R. & Ager, A. Activation of pertussis toxin-sensitive CXCL12 (SDF-1) receptors mediates transendothelial migration of T lymphocytes across lymph node high endothelial cells. Eur. J. Immunol. 32, 837–847 (2002).
Cinamon, G., Shinder, V. & Alon, R. Shear forces promote lymphocyte migration across vascular endothelium bearing apical chemokines. Nature Immunol. 2, 515–522 (2001).
Yamamoto, J. et al. Differential expression of the chemokine receptors by the TH1- and TH2-type effector populations within circulating CD4+ T cells. J. Leukoc. Biol. 68, 568–574 (2000).
Nagakubo, D. et al. A high endothelial venule secretory protein, mac25/angiomodulin, interacts with multiple high endothelial venule-associated molecules including chemokines. J. Immunol. 171, 553–561 (2003). The first study to show that a component of the basal lamina of HEVs can bind lymphoid chemokines preferentially and present them to receptor-expressing cells.
Janatpour, M. J. et al. Tumor necrosis factor-dependent segmental control of MIG expression by high endothelial venules in inflamed lymph nodes regulates monocyte recruitment. J. Exp. Med. 194, 1375–1384 (2001).
Loetscher, M. et al. Chemokine receptor specific for IP10 and MIG: structure, function, and expression in activated T lymphocytes. J. Exp. Med. 184, 963–969 (1996).
Yoshida, R. et al. EBI1-ligand chemokine (ELC) attracts a broad spectrum of lymphocytes: activated T cells strongly up-regulate CCR7 and efficiently migrate toward ELC. Int. Immunol. 10, 901–910 (1998).
Nagira, M. et al. A lymphocyte-specific CC chemokine, secondary lymphoid tissue chemokine (SLC), is a highly efficient chemoattractant for B cells and activated T cells. Eur. J. Immunol. 28, 1516–1523 (1998).
Warnock, R. A. et al. The role of chemokines in the microenvironmental control of T versus B cell arrest in Peyer's patch high endothelial venules. J. Exp. Med. 191, 77–88 (2000). The authors use intravital microscopy to show the presence of T- and B-lymphotropic HEVs. This paper also shows that CCL21 expression by HEVs closely correlates with preferential T-cell adhesion to HEVs.
Forster, R., Emrich, T., Kremmer, E. & Lipp, M. Expression of the G-protein-coupled receptor BLR1 defines mature, recirculating B cells and a subset of T-helper memory cells. Blood 84, 830–840 (1994).
Gunn, M. D. et al. A B-cell homing chemokine made in lymphoid follicles activates Burkitt's lymphoma receptor-1. Nature 391, 799–803 (1998).
Legler, D. F. et al. B cell-attracting chemokine 1, a human CXC chemokine expressed in lymphoid tissues, selectively attracts B lymphocytes via BLR1/CXCR5. J. Exp. Med. 187, 655–660 (1998).
Gretz, J. E., Kaldjian, E., Anderson, A. O. & Shaw, S. Sophisticated strategies for information encounter in the lymph node: the reticular network as a conduit of soluble information and a highway for cell traffic. J. Immunol. 157, 495–499 (1996).
Gretz, J. E. et al. Lymph-borne chemokines and other low molecular weight molecules reach high endothelial venules via specialized conduits while a functional barrier limits access to the lymphocyte microenvironments in lymph node cortex. J. Exp. Med. 192, 1425–1440 (2000).
Ford, W. L. Lymphocyte migration and immune responses. Prog. Allergy 19, 1–59 (1975).
Reif, K. et al. Balanced responsiveness to chemoattractants from adjacent zones determines B-cell position. Nature 416, 94–99 (2002).
Gutman, G. A. & Weissman, I. L. Homing properties of thymus-independent follicular lymphocytes. Transplantation 16, 621–629 (1973).
Nieuwenhuis, P. & Ford, W. L. Comparative migration of B- and T-lymphocytes in the rat spleen and lymph nodes. Cell. Immunol. 23, 254–267 (1976).
Palframan, R. et al. Inflammatory chemokine transport and presentation in HEV: a remote control mechanism for monocyte recruitment to lymph nodes in inflamed tissues. J. Exp. Med. 194, 1361–1373 (2001).
Muller, G. & Lipp, M. Shaping up adaptive immunity: the impact of CCR7 and CXCR5 on lymphocyte trafficking. Microcirculation 10, 325–334 (2003).
Willimann, K. et al. The chemokine SLC is expressed in T cell areas of lymph nodes and mucosal lymphoid tissues and attracts activated T cells via CCR7. Eur. J. Immunol. 28, 2025–2034 (1998).
Ansel, K. M. et al. In vivo-activated CD4 T cells upregulate CXC chemokine receptor 5 and reprogram their response to lymphoid chemokines. J. Exp. Med. 190, 1123–1134 (1999).
Schaerli, P. et al. CXC chemokine receptor 5 expression defines follicular homing T cells with B cell helper function. J. Exp. Med. 192, 1553–1562 (2000).
Breitfeld, D. et al. Follicular B helper T cells express CXC chemokine receptor 5, localize to B cell follicles, and support immunoglobulin production. J. Exp. Med. 192, 1545–1552 (2000).
Sallusto, F., Palermo, B., Hoy, A. & Lanzavecchia, A. The role of chemokine receptors in directing traffic of naive, type 1 and type 2 T cells. Curr. Top. Microbiol. Immunol. 246, 123–128 (1999).
Sallusto, F., Mackay, C. R. & Lanzavecchia, A. The role of chemokine receptors in primary, effector, and memory immune responses. Annu. Rev. Immunol. 18, 593–620 (2000).
Goodarzi, K. et al. Leukotriene B4 and BLT1 control cytotoxic effector T cell recruitment to inflamed tissues. Nature Immunol. 4, 965–973 (2003).
Ott, V. L. et al. Mast-cell dependent migration of effector CD8+ T cells through production of leukotriene B4 . Nature Immunol. 4, 974–981 (2003).
Tager, A. M. et al. Leukotriene B4 receptor BLT1 mediates early effector T cell recruitment. Nature Immunol. 4, 982–990 (2003).
Bessis, M. in Living Blood Cells and Their Ultrastructure 543 (Springer, Berlin, 1973).
Middleton, J. et al. Transcytosis and surface presentation of IL-8 by venular endothelial cells. Cell 91, 385–395 (1997).
Nolte, M. A. et al. A conduit system distributes chemokines and small blood-borne molecules through the splenic white pulp. J. Exp. Med. 198, 505–512 (2003).
Foxman, E. F., Campbell, J. J. & Butcher, E. C. Multistep navigation and the combinatorial control of leukocyte chemotaxis. J. Cell Biol. 139, 1349–1360 (1997).
Wei, S. H., Parker, I., Miller, M. J. & Cahalan, M. D. A stochastic view of lymphocyte motility and trafficking within the lymph node. Immunol. Rev. 195, 136–159 (2003). This paper indicates that lymphocytes might respond to matrix-immobilized chemokines.
Wilkinson, P. C. Assays of leukocyte locomotion and chemotaxis. J. Immunol. Methods 216, 139–153 (1998).
Bernfield, M. et al. Functions of cell surface heparan sulfate proteoglycans. Annu. Rev. Biochem. 68, 729–777 (1999).
Hirose, J. et al. Versican interacts with chemokines and modulates cellular responses. J. Biol. Chem. 276, 5228–5234 (2001).
Kawashima, H. et al. Oversulfated chondroitin/dermatan sulfates containing GlcAβ1/IdoAα1-3GalNAc(4,6-O-disulfate) interacts with L- and P-selectin and chemokines. J. Biol. Chem. 277, 12921–12930 (2002).
Izawa, D. et al. Expression profile of active genes in mouse lymph node high endothelial cells. Int. Immunol. 11, 1989–1998 (1999).
Cheifetz, S. et al. Endoglin is a component of the transforming growth factor-β receptor system in human endothelial cells. J. Biol. Chem. 267, 19027–19030 (1992).
Saito, K. et al. Gene expression profiling of mucosal addressin cell adhesion molecule-1+ high endothelial venule cells (HEV) and identification of a leucine-rich HEV glycoprotein as a HEV marker. J. Immunol. 168, 1050–1059 (2002).
Chin, Y. -H., Ye, M. -W., Cai, J. -P. & Wu, X. -M. Differential regulation of tissue-specific lymph node high endothelial venule cell adhesion molecules by tumor necrosis factor and transforming growth factor-β1. Immunology 87, 559–565 (1996).
Girard, J. -P. et al. Heterogeneity of endothelial cells. The specialized phenotype of human high endothelial venules characterized by suppression subtractive hybridization. Am. J. Pathol. 155, 2043–2055 (1999).
Kashiwazaki, M. et al. A high endothelial venule-expressing promiscuous chemokine receptor DARC can bind inflammatory, but not lymphoid, chemokines and is dispensable for lymphocyte homing under physiological conditions. Int. Immunol. 15, 1219–1227 (2003).
Neote, K., Mak, J. Y., Kolakowski, L. F. Jr & Schall, T. J. Functional and biochemical analysis of the cloned Duffy antigen: identity with the red blood cell chemokine receptor. Blood 84, 44–52 (1994).
Hadley, T. J. & Peiper, S. C. From malaria to chemokine receptor: the emerging physiologic role of the Duffy blood group antigen. Blood 89, 3077–3091 (1997).
Baxter, R. C. et al. Recommendations for nomenclature of the insulin-like growth factor binding protein superfamily. Endocrinology 139, 4036 (1998).
Akaogi, K. et al. Specific accumulation of tumor-derived adhesion factor in tumor blood vessels and in capillary tube-like structures of cultured vascular endothelial cells. Proc. Natl Acad. Sci. USA 93, 8384–8389 (1996).
Sato, J. et al. Identification of cell-binding site of angiomodulin (AGM/TAF/Mac25) that interacts with heparan sulfates on cell surface. J. Cell. Biochem. 75, 187–195 (1999).
Hwa, V., Oh, Y. & Rosenfeld, R. G. The insulin-like growth factor-binding protein (IGFBP) superfamily. Endocr. Rev. 20, 761–787 (1999).
Usui, T. et al. Characterization of mac25/angiomodulin expression by high endothelial venule cells in lymphoid tissues and its identification as an inducible marker for activated endothelial cells. Int. Immunol. 14, 1273–1282 (2002).
Pelletier, A. J. et al. Presentation of chemokine SDF-1α by fibronectin mediates directed migration of T cells. Blood 96, 2682–2690 (2000).
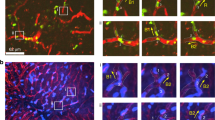
Miller, M. J., Wei, S. H., Cahalan, M. D. & Parker, I. Autonomous T cell trafficking examined in vivo with intravital two-photon microscopy. Proc. Natl Acad. Sci. USA 100, 2604–2609 (2003). Together with reference 99, this paper shows that, after extravasation through HEVs, T cells move rapidly in the lymph-node parenchyma in a seemingly random manner.
Miller, M. J. et al. T cell repertoire scanning is promoted by dynamic dendritic cell behavior and random T cell motility in the lymph node. Proc. Natl Acad. Sci. USA 101, 998–1003 (2004).
Twisk, A. J., Groeneveld, P. H. & Kraal, G. The effects of bacterial lipopolysaccharide (LPS) on high endothelial venules and interdigitating cells in mouse lymph nodes. Immunobiology 176, 410–422 (1988).
Baekkevold, E. S. et al. Molecular characterization of NF-HEV, a nuclear factor preferentially expressed in human high endothelial venules. Am. J. Pathol. 163, 69–79 (2003).
Streeter, P. R., Rouse, B. T. & Butcher, E. C. Immunohistologic and functional characterization of a vascular addressin involved in lymphocyte homing into peripheral lymph nodes. J. Cell Biol. 107, 1853–1862 (1988).
Cahill, R. N. P., Poskitt, D., Frost, H. & Trnka, Z. Two distinct pools of recirculating T lymphocytes: migratory characteristics of nodal and intestinal T lymphocytes. J. Exp. Med. 145, 420–428 (1977).
Streeter, P. R. et al. A tissue-specific endothelial cell molecule involved in lymphocyte homing. Nature 331, 41–46 (1988).
Nakache, M., Berg, E. L., Streeter, P. R. & Butcher, E. C. The mucosal vascular addressin is a tissue-specific endothelial cell adhesion molecule for circulating lymphocytes. Nature 337, 179–181 (1989).
Stevens, S. K., Weissman, I. L. & Butcher, E. C. Differences in the migration of B and T lymphocytes: organ-selective localization in vivo and the role of lymphocyte-endothelial cell recognition. J. Immunol. 128, 844–851 (1982).
Sprent, J. Circulating T and B lymphocytes of the mouse. I. Migratory properties. Cell. Immunol. 7, 10–39 (1973).
Westermann, J. et al. IFN-γ influences the migration of thoracic duct B and T lymphocyte subsets in vivo. Random increase in disappearance from the blood and differential decrease in reappearance in the lymph. J. Immunol. 150, 3843–3852 (1993).
Blaschke, V., Micheel, B., Pabst, R. & Westermann, J. Lymphocyte traffic through lymph nodes and Peyer's patches of the rat: B- and T-cell-specific migration patterns within the tissue, and their dependence on splenic tissue. Cell Tissue Res. 282, 377–386 (1995).
Kim, C. H. et al. Rules of chemokine receptor association with T cell polarization in vivo. J. Clin. Invest. 108, 1331–1339 (2001).
Debes, G. F., Hopken, U. E. & Hamann, A. In vivo differentiated cytokine-producing CD4+ T cells express functional CCR7. J. Immunol. 168, 5441–5447 (2002).
Acknowledgements
We thank R. Pabst, J. Westermann and T. Hirata for critically reading the manuscript. We also thank members of the Laboratory of Molecular and Cellular Recognition for discussions and suggestions. We thank the Japanese Ministry of Education, Culture, Sports, Science and Technology for continuous grant support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- PEYER'S PATCHES
-
Groups of lymphoid nodules that are present in the small intestine (usually the ileum). They occur massed together on the intestinal wall, opposite the line of attachment of the mesentery. Peyer's patches consist of a dome area, B-cell follicles and interfollicular T-cell areas. High endothelial venules are present mainly in the interfollicular areas.
- HIGH ENDOTHELIAL VENULES
-
(HEVs). Venules (small veins that join capillaries to larger veins) that have a high-walled endothelium and are present in the paracortex of lymph nodes and tonsils, as well as in the interfollicular areas of Peyer's patches.
- BASAL LAMINA
-
A supporting structure located at the boundary between endothelia (or epithelia) and the underlying connective tissue. In blood vessels, the basal lamina surrounds the endothelial cells and pericytes, providing physical support. It is composed mainly of collagen IV and laminin molecules. Fibronectin is also present on the connective-tissue face. The basal lamina also seems to be involved in maintaining and modulating endothelial-cell functions by capturing various humoral factors.
- INTERFOLLICULAR HEVS
-
High endothelial venules (HEVs) located in the interfollicular area — the T-cell-dependent area of the lymph-node cortex, which lies between B-cell follicles. Most HEVs belong to this category.
- FOLLICULAR HEVS
-
High endothelial venules (HEVs) located within B-cell follicles.
- PARAFOLLICULAR HEVS
-
High endothelial venules (HEVs) found near, but not within, B-cell follicles.
- DRY MOTIF
-
An amino-acid motif composed of aspartic acid (D), arginine (R) and tyrosine (Y). It is highly conserved among G-protein-coupled receptors and is thought to be essential for G-protein-mediated signalling.
- INTRAVITAL MULTI-PHOTON LASER MICROSCOPY
-
Multi-photon laser microscopy combines the advanced optical techniques of laser-scanning confocal microscopy with long-wavelength multi-photon fluorescence excitation to capture high-resolution, three-dimensional images of living cells and/or tissues that have been labelled with fluorophores. It provides a greater tissue imaging depth (up to 350 μm depending on the tissue) and less photobleaching and phototoxicity than conventional imaging methods.
Rights and permissions
About this article
Cite this article
Miyasaka, M., Tanaka, T. Lymphocyte trafficking across high endothelial venules: dogmas and enigmas. Nat Rev Immunol 4, 360–370 (2004). https://doi.org/10.1038/nri1354
Issue Date:
DOI: https://doi.org/10.1038/nri1354
This article is cited by
-
Reprogramming of sentinel lymph node microenvironment during tumor metastasis
Journal of Biomedical Science (2022)
-
The role of lymphatics in intestinal inflammation
Inflammation and Regeneration (2021)
-
High endothelial venules (HEVs) in immunity, inflammation and cancer
Angiogenesis (2021)
-
Robo4 contributes to the turnover of Peyer's patch B cells
Mucosal Immunology (2020)
-
Prolonged residence of an albumin–IL-4 fusion protein in secondary lymphoid organs ameliorates experimental autoimmune encephalomyelitis
Nature Biomedical Engineering (2020)