Key Points
-
During sterile injury or infection, the innate immune system produces cytokines as part of the innate immune response.
-
Molecules such as the cytokines tumour necrosis factor and interleukin-1 as well as high-mobility group box 1 protein are inherently toxic and can directly mediate the development of fever, shock, tissue injury, organ failure and even death.
-
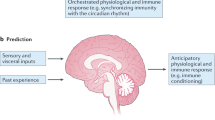
Visceral reflexes provide physiological homeostasis to complex organ systems (for example, the baroreflex in the cardiovascular system), and these principles also operate in the control of innate immune responses.
-
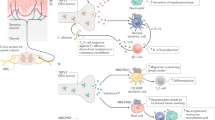
The inflammatory reflex, a prototypical neural mechanism that modulates the immune system, consists of an afferent sensory arm that is activated by the products of sterile or infectious inflammation, and a motor arm, termed the cholinergic anti-inflammatory pathway, that inhibits pro-inflammatory cytokine release by the innate immune system.
-
The molecular mechanism of cytokine regulation by the inflammatory reflex depends on signal transduction by the nicotinic acetylcholine receptor α7 subunit (α7nAChR).
-
Administration of selective α7nAChR agonists or electrical stimulation of the inflammatory reflex inhibits pro-inflammatory cytokine release and confers protection against tissue damage.
-
The immune system is not autonomous because immune responses are influenced by the inflammatory reflex.
-
The inflammatory reflex maintains immune homeostasis as a set point function that influences the progression of inflammatory diseases.
Abstract
Inflammation can cause damage and even death. What controls this primitive and potentially lethal innate immune response to injury and infection? Molecular and neurophysiological studies during the past decade have revealed a pivotal answer: immunity is coordinated by neural circuits that operate reflexively. The afferent arc of the reflex consists of nerves that sense injury and infection. This activates efferent neural circuits, including the cholinergic anti-inflammatory pathway, that modulate immune responses and the progression of inflammatory diseases. It might be possible to develop therapeutics that target neural networks for the treatment of inflammatory disorders.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Tracey, K. J. The inflammatory reflex. Nature 420, 853–859 (2002).
Borovikova, L. V. et al. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 405, 458–462 (2000).
Tracey, K. J. Physiology and immunology of the cholinergic antiinflammatory pathway. J. Clin. Invest 117, 289–296 (2007).
Steinman, L. Elaborate interactions between the immune and nervous systems. Nature Immunol. 5, 575–581 (2004).
Sternberg, E. M. Neural regulation of innate immunity: a coordinated nonspecific host response to pathogens. Nature Rev. Immunol. 6, 318–328 (2006).
Bellinger, D. L. et al. Sympathetic modulation of immunity: relevance to disease. Cell Immunol. 252, 27–56 (2008).
del Rey, A. & Besedovsky, H. O. Sympathetic nervous system–immune interactions in autoimmune lymphoproliferative diseases. Neuroimmunomodulation 15, 29–36 (2008).
Blalock, J. E. & Smith, E. M. Conceptual development of the immune system as a sixth sense. Brain Behav. Immun. 21, 23–33 (2007).
Albuquerque, E. X., Pereira, E. F., Alkondon, M. & Rogers, S. W. Mammalian nicotinic acetylcholine receptors: from structure to function. Physiol. Rev. 89, 73–120 (2009).
van Koppen, C. J. & Kaiser, B. Regulation of muscarinic acetylcholine receptor signaling. Pharmacol. Ther. 98, 197–220 (2003).
Wess, J., Eglen, R. M. & Gautam, D. Muscarinic acetylcholine receptors: mutant mice provide new insights for drug development. Nature Rev. Drug Discov. 6, 721–733 (2007).
Koizumi, K., Terui, N., Kollai, M. & Brooks, C. M. Functional significance of coactivation of vagal and sympathetic cardiac nerves. Proc. Natl Acad. Sci. USA 79, 2116–2120 (1982).
Dinarello, C. A. et al. Tumor necrosis factor (cachectin) is an endogenous pyrogen and induces production of interleukin 1. J. Exp. Med. 163, 1433–1450 (1986).
Tracey, K. J. et al. Metabolic effects of cachectin/tumor necrosis factor are modified by site of production. Cachectin/tumor necrosis factor-secreting tumor in skeletal muscle induces chronic cachexia, while implantation in brain induces predominantly acute anorexia. J. Clin. Invest 86, 2014–2024 (1990).
Watkins, L. R. & Maier, S. F. Immune regulation of central nervous system functions: from sickness responses to pathological pain. J. Intern. Med. 257, 139–155 (2005).
Dantzer, R., O'Connor, J. C., Freund, G. G., Johnson, R. W. & Kelley, K. W. From inflammation to sickness and depression: when the immune system subjugates the brain. Nature Rev. Neurosci. 9, 46–56 (2008).
Davis, C. N. et al. MyD88-dependent and -independent signaling by IL-1 in neurons probed by bifunctional Toll/IL-1 receptor domain/BB-loop mimetics. Proc. Natl Acad. Sci. USA 103, 2953–2958 (2006).
Mattson, M. P. NF-κB in the survival and plasticity of neurons. Neurochem. Res. 30, 883–893 (2005).
Ferri, C. C., Yuill, E. A. & Ferguson, A. V. Interleukin-1β depolarizes magnocellular neurons in the paraventricular nucleus of the hypothalamus through prostaglandin-mediated activation of a non selective cationic conductance. Regul. Pept. 129, 63–71 (2005).
Watkins, L. R. et al. Blockade of interleukin-1 induced hyperthermia by subdiaphragmatic vagotomy: evidence for vagal mediation of immune–brain communication. Neurosci. Lett. 183, 27–31 (1995).
Hansen, M. K., O'Connor, K. A., Goehler, L. E., Watkins, L. R. & Maier, S. F. The contribution of the vagus nerve in interleukin-1β-induced fever is dependent on dose. Am. J. Physiol. Regul. Integr. Comp. Physiol. 280, R929–R934 (2001).
Goehler, L. E. et al. Vagal paraganglia bind biotinylated interleukin-1 receptor antagonist: a possible mechanism for immune-to-brain communication. Brain Res. Bull. 43, 357–364 (1997).
Wang, H. Y. & Fitzgerald, R. S. Muscarinic modulation of hypoxia-induced release of catecholamines from the cat carotid body. Brain Res. 927, 122–137 (2002).
Neubauer, J. A. & Sunderram, J. Oxygen-sensing neurons in the central nervous system. J. Appl. Physiol. 96, 367–374 (2004).
Goehler, L. E. et al. Interleukin-1beta in immune cells of the abdominal vagus nerve: a link between the immune and nervous systems? J. Neurosci. 19, 2799–2806 (1999).
Niijima, A. The afferent discharges from sensors for interleukin 1β in the hepatoportal system in the anesthetized rat. J. Auton. Nerv. Syst. 61, 287–291 (1996).
Medzhitov, R. Origin and physiological roles of inflammation. Nature 454, 428–435 (2008).
Bianchi, M. E. DAMPs, PAMPs and alarmins: all we need to know about danger. J. Leukoc. Biol. 81, 1–5 (2007).
Nathan, C. Points of control in inflammation. Nature 420, 846–852 (2002).
Wang, H. et al. Nicotinic acetylcholine receptor α7 subunit is an essential regulator of inflammation. Nature 421, 384–388 (2003).
Ghia, J. E., Blennerhassett, P. & Collins, S. M. Impaired parasympathetic function increases susceptibility to inflammatory bowel disease in a mouse model of depression. J. Clin. Invest 118, 2209–2218 (2008).
Ghia, J. E., Blennerhassett, P., Kumar-Ondiveeran, H., Verdu, E. F. & Collins, S. M. The vagus nerve: a tonic inhibitory influence associated with inflammatory bowel disease in a murine model. Gastroenterology 131, 1122–1130 (2006).
van Westerloo, D. J. et al. The vagus nerve and nicotinic receptors modulate experimental pancreatitis severity in mice. Gastroenterology 130, 1822–1830 (2006).
van Westerloo, D. J. et al. The cholinergic anti-inflammatory pathway regulates the host response during septic peritonitis. J. Infect. Dis. 191, 2138–2148 (2005).
Guarini, S. et al. Efferent vagal fibre stimulation blunts nuclear factor-κB activation and protects against hypovolemic hemorrhagic shock. Circulation 107, 1189–1194 (2003).
Bernik, T. R. et al. Pharmacological stimulation of the cholinergic antiinflammatory pathway. J. Exp. Med. 195, 781–788 (2002).
Saeed, R. W. et al. Cholinergic stimulation blocks endothelial cell activation and leukocyte recruitment during inflammation. J. Exp. Med. 201, 1113–1123 (2005).
Huston, J. M. et al. Splenectomy inactivates the cholinergic antiinflammatory pathway during lethal endotoxemia and polymicrobial sepsis. J. Exp. Med. 203, 1623–1628 (2006).
Charpantier, E. et al. α7 neuronal nicotinic acetylcholine receptors are negatively regulated by tyrosine phosphorylation and Src-family kinases. J. Neurosci. 25, 9836–9849 (2005).
Kihara, T. et al. α7 nicotinic receptor transduces signals to phosphatidylinositol 3-kinase to block A β-amyloid-induced neurotoxicity. J. Biol. Chem. 276, 13541–13546 (2001).
de Jonge, W. J. et al. Stimulation of the vagus nerve attenuates macrophage activation by activating the Jak2–STAT3 signaling pathway. Nature Immunol. 6, 844–851 (2005).
Dajas-Bailador, F. A., Soliakov, L. & Wonnacott, S. Nicotine activates the extracellular signal-regulated kinase 1/2 via the α7 nicotinic acetylcholine receptor and protein kinase A, in SH-SY5Y cells and hippocampal neurones. J. Neurochem. 80, 520–530 (2002).
Wang, H. et al. Cholinergic agonists inhibit HMGB1 release and improve survival in experimental sepsis. Nature Med. 10, 1216–1221 (2004).
Parrish, W. R. et al. Modulation of TNF release by choline requires α7 subunit nicotinic acetylcholine receptor-mediated signaling. Mol. Med. 14, 567–574 (2008).
Pavlov, V. A. et al. Selective α7-nicotinic acetylcholine receptor agonist GTS-21 improves survival in murine endotoxemia and severe sepsis. Crit. Care Med. 35, 1139–1144 (2007).
Rosas-Ballina, M. et al. Splenic nerve is required for cholinergic antiinflammatory pathway control of TNF in endotoxemia. Proc. Natl Acad. Sci. USA 105, 11008–11013 (2008).
Hamano, R. et al. Stimulation of α7 nicotinic acetylcholine receptor inhibits CD14 and the Toll-like receptor 4 expression in human monocytes. Shock 26, 358–364 (2006).
Yoshikawa, H. et al. Nicotine inhibits the production of proinflammatory mediators in human monocytes by suppression of I-κB phosphorylation and nuclear factor-κB transcriptional activity through nicotinic acetylcholine receptor α7. Clin. Exp. Immunol. 146, 116–123 (2006).
Suzuki, T. et al. Microglial α7 nicotinic acetylcholine receptors drive a phospholipase C/IP3 pathway and modulate the cell activation toward a neuroprotective role. J. Neurosci. Res. 83, 1461–1470 (2006).
Shytle, R. D. et al. Cholinergic modulation of microglial activation by α7 nicotinic receptors. J. Neurochem. 89, 337–343 (2004).
Razani-Boroujerdi, S. et al. T cells express α7-nicotinic acetylcholine receptor subunits that require a functional TCR and leukocyte-specific protein tyrosine kinase for nicotine-induced Ca2+ response. J. Immunol. 179, 2889–2898 (2007).
Takahashi, H. K. et al. The immunosuppressive effects of nicotine during human mixed lymphocyte reaction. Eur. J. Pharmacol. 559, 69–74 (2007).
Berthoud, H. R. & Powley, T. L. Characterization of vagal innervation to the rat celiac, suprarenal and mesenteric ganglia. J. Auton. Nerv. Syst. 42, 153–169 (1993).
Berthoud, H. R. & Powley, T. L. Interaction between parasympathetic and sympathetic nerves in prevertebral ganglia: morphological evidence for vagal efferent innervation of ganglion cells in the rat. Microsc. Res. Tech. 35, 80–86 (1996).
Bellinger, D. L., Lorton, D., Hamill, R. W., Felten, S. Y. & Felten, D. L. Acetylcholinesterase staining and choline acetyltransferase activity in the young adult rat spleen: lack of evidence for cholinergic innervation. Brain Behav. Immun. 7, 191–204 (1993).
Bellinger, D. L., Felten, S. Y., Lorton, D. & Felten, D. L. Origin of noradrenergic innervation of the spleen in rats. Brain Behav. Immun. 3, 291–311 (1989).
Brandon, K. W. & Rand, M. J. Acetylcholine and the sympathetic innervation of the spleen. J. Physiol. 157, 18–32 (1961).
Leaders, F. E. & Dayrit, C. The cholinergic component in the sympathetic innervation to the spleen. J. Pharmacol. Exp. Ther. 147, 145–152 (1965).
Bulloch, K., Damavandy, T. & Badamchian, M. Characterization of choline O-acetyltransferase (ChAT) in the BALB/C mouse spleen. Int. J. Neurosci. 76, 141–149 (1994).
Kawashima, K. & Fujii, T. Expression of non-neuronal acetylcholine in lymphocytes and its contribution to the regulation of immune function. Front Biosci. 9, 2063–2085 (2004).
Kawashima, K. & Fujii, T. The lymphocytic cholinergic system and its contribution to the regulation of immune activity. Life Sci. 74, 675–696 (2003).
von Kanel, R., Nelesen, R. A., Mills, P. J., Ziegler, M. G. & Dimsdale, J. E. Relationship between heart rate variability, interleukin-6, and soluble tissue factor in healthy subjects. Brain Behav. Immun. 22, 461–468 (2008).
Haensel, A., Mills, P. J., Nelesen, R. A., Ziegler, M. G. & Dimsdale, J. E. The relationship between heart rate variability and inflammatory markers in cardiovascular diseases. Psychoneuroendocrinology 33, 1305–1312 (2008).
Marsland, A. L. et al. Stimulated production of proinflammatory cytokines covaries inversely with heart rate variability. Psychosom. Med. 69, 709–716 (2007).
Holguin, F. et al. Cardiac autonomic changes associated with fish oil vs soy oil supplementation in the elderly. Chest 127, 1102–1107 (2005).
Lanza, G. A. et al. Relation of heart rate variability to serum levels of C-reactive protein in patients with unstable angina pectoris. Am. J. Cardiol. 97, 1702–1706 (2006).
Jae, S. Y. et al. Effects of lifestyle modifications on C-reactive protein: contribution of weight loss and improved aerobic capacity. Metabolism 55, 825–831 (2006).
Goldhammer, E. et al. Exercise training modulates cytokines activity in coronary heart disease patients. Int. J. Cardiol. 100, 93–99 (2005).
Sloan, R. P. et al. Aerobic exercise attenuates inducible TNF production in humans. J. Appl. Physiol 103, 1007–1011 (2007).
Thayer, J. F. & Fischer, J. E. Heart rate variability, overnight urinary norepinephrine and C-reactive protein: evidence for the cholinergic anti-inflammatory pathway in healthy human adults. J. Intern. Med. 265, 439–447 (2008).
Thayer, J. F. & Lane, R. D. The role of vagal function in the risk for cardiovascular disease and mortality. Biol. Psychol. 74, 224–242 (2007).
Thayer, J. F. & Sternberg, E. Beyond heart rate variability: vagal regulation of allostatic systems. Ann. NY Acad. Sci. 1088, 361–372 (2006).
Stein, K. S., McFarlane, I. C., Goldberg, N. & Ginzler, E. M. Heart rate variability in patients with systemic lupus erythematosus. Lupus 5, 44–48 (1996).
Barnaby, D. et al. Heart rate variability in emergency department patients with sepsis. Acad. Emerg. Med. 9, 661–670 (2002).
Pontet, J. et al. Heart rate variability as early marker of multiple organ dysfunction syndrome in septic patients. J. Crit. Care 18, 156–163 (2003).
Evrengul, H. et al. Heart rate variability in patients with rheumatoid arthritis. Rheumatol. Int. 24, 198–202 (2004).
Laversuch, C. J. et al. Reduction in heart rate variability in patients with systemic lupus erythematosus. J. Rheumatol. 24, 1540–1544 (1997).
Lindgren, S., Stewenius, J., Sjolund, K., Lilja, B. & Sundkvist, G. Autonomic vagal nerve dysfunction in patients with ulcerative colitis. Scand. J. Gastroenterol. 28, 638–642 (1993).
Uslu, N. et al. Heart rate variability in patients with systemic sarcoidosis. Ann. Noninvasive Electrocardiol. 11, 38–42 (2006).
Biswas, A. K., Scott, W. A., Sommerauer, J. F. & Luckett, P. M. Heart rate variability after acute traumatic brain injury in children. Crit. Care Med. 28, 3907–3912 (2000).
Hanson, G. K. & Libby, P. The immune response in atherosclerosis: a double-edged sword. Nature Rev. Immunol. 6, 508–519 (2006).
Sloan, R. P. et al. RR interval variability is inversely related to inflammatory markers: the CARDIA study. Mol. Med. 13, 178–184 (2007).
Bhatia, M., Zhi, L., Zhang, H., Ng, S. W. & Moore, P. K. Role of substance P in hydrogen sulfide-induced pulmonary inflammation in mice. Am. J. Physiol. Lung Cell Mol. Physiol. 291, L896–L904 (2006).
Spengler, R. N., Chensue, S. W., Giacherio, D. A., Blenk, N. & Kunkel, S. L. Endogenous norepinephrine regulates tumor necrosis factor-alpha production from macrophages in vitro. J. Immunol. 152, 3024–3031 (1994).
Yu, B. et al. Serotonin 5-hydroxytryptamine2A receptor activation suppresses tumor necrosis factor-α-induced inflammation with extraordinary potency. J. Pharmacol. Exp. Ther. 327, 316–323 (2008).
Yoshimura, T. et al. Inhibition of tumor necrosis factor-alpha and interleukin-1-beta production by beta-adrenoceptor agonists from lipopolysaccharide-stimulated human peripheral blood mononuclear cells. Pharmacology 54, 144–152 (1997).
Pavlov, V. A. et al. Central muscarinic cholinergic regulation of the systemic inflammatory response during endotoxemia. Proc. Natl Acad. Sci. USA 103, 5219–5223 (2006).
Pavlov, V. A. et al. Brain acetylcholinesterase activity controls systemic cytokine levels through the cholinergic anti-inflammatory pathway. Brain Behav. Immun. 23, 41–45 (2009).
Labiner, D. M. & Ahern, G. L. Vagus nerve stimulation therapy in depression and epilepsy: therapeutic parameter settings. Acta Neurol. Scand. 115, 23–33 (2007).
DeGiorgio, C. M. et al. Prospective long-term study of vagus nerve stimulation for the treatment of refractory seizures. Epilepsia 41, 1195–1200 (2000).
Sanders, V. M. & Straub, R. H. Norepinephrine, the β-adrenergic receptor, and immunity. Brain Behav. Immun. 16, 290–332 (2002).
Saravia, F. & Homo-Delarche, F. Is innervation an early target in autoimmune diabetes? Trends Immunol. 24, 574–579 (2003).
Straub, R. H., Rauch, L., Fassold, A., Lowin, T. & Pongratz, G. Neuronally released sympathetic neurotransmitters stimulate splenic interferon-γ secretion from T cells in early type II collagen-induced arthritis. Arthritis Rheum. 58, 3450–3460 (2008).
van Maanen, M. A. et al. Stimulation of nicotinic acetylcholine receptors attenuates collagen-induced arthritis in mice. Arthritis Rheum. 60, 114–122 (2009).
Zhang, P., Han, D., Tang, T., Zhang, X. & Dai, K. Inhibition of the development of collagen-induced arthritis in Wistar rats through vagus nerve suspension: a 3-month observation. Inflamm. Res. 57, 322–328 (2008).
Dampney, R. A. et al. Long-term regulation of arterial blood pressure by hypothalamic nuclei: some critical questions. Clin. Exp. Pharmacol. Physiol. 32, 419–425 (2005).
Huston, J. M. et al. Transcutaneous vagus nerve stimulation reduces serum high mobility group box 1 levels and improves survival in murine sepsis. Crit. Care Med. 35, 2762–2768 (2007).
Kawada, T. et al. Vagal stimulation suppresses ischemia-induced myocardial interstitial myoglobin release. Life Sci. 83, 490–495 (2008).
Ghia, J. E., Blennerhassett, P., El Sharkawy, R. T. & Collins, S. M. The protective effect of the vagus nerve in a murine model of chronic relapsing colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 293, G711–G718 (2007).
Ghia, J. E., Blennerhassett, P. & Collins, S. M. Vagus nerve integrity and experimental colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 293, G560–G567 (2007).
Acknowledgements
The author thanks S. Chavan, M. Rosas-Ballina, V. Pavlov, B. Volpe, P. Huerta, B. Diamond and S. Warren for helpful discussions. Work in the author's laboratory was supported in part by the National Institute of General Medical Sciences, one of the National Institutes of Health.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
K. Tracey is an inventor on patents related to the cholinergic anti-inflammatory pathway and is a consultant on this topic.
Related links
Glossary
- Sickness syndrome
-
The physiological and behavioural responses to the molecular products of infection or injury that can be triggered by the accumulation of pro-inflammatory cytokines in the brain. These include (but are not limited to) fever, acute phase responses, anorexia, weight loss, increased sleep, decreased social interaction, exploration, decreased sexual activity and altered hypothalamic, pituitary and autonomic output.
- Pyrogen
-
An agent that is derived from either a pathogen or the host and that can cause fever in vivo. Examples of pyrogens include bacterial endotoxin and cytokines, such as tumour necrosis factor, interleukin-1 (IL-1) and IL-6.
- Glomus cell
-
A type of epithelial cell located in the carotid body or in clusters (termed paraganglia) adjacent to the vagus and other autonomic nerves. A glomus cell can be activated by chemical changes in the cellular milieu to release dopamine or other neurotransmitters close to nerves.
- Pathogen-associated molecular pattern
-
A microbial motif that can stimulate innate immune responses; for example, bacterial endotoxins, peptidoglycan, flagellin, double-stranded RNA and unmethylated CpG-containing DNA.
- Toll-like receptor
-
A membrane receptor that is expressed by innate immune cells and that interacts with molecular products of infection or injury to initiate cytokine release and trigger inflammation.
- Damage-associated molecular pattern
-
A molecule that is produced by the host and that can stimulate innate immune responses; for example, high-mobility group box protein, uric acid, heat shock proteins, heparin sulphate, hyaluronan fragments, ATP and DNA.
- Nucleotide-binding oligiomerization domain (NOD)-like receptor
-
An intracellular pattern recognition receptor belonging to a family comprising 20 members that recognize endogenous or exogenous molecular products of infection or injury, several of which activate caspases that activate cytokine release and nuclear factor-κB signalling.
- Sepsis
-
A systemic inflammatory syndrome that occurs after infection or injury and is characterized by a constellation of symptoms, including, but not limited to, alterations in body temperature, white blood cell count, heart rate, respiratory rate and organ function.
- Catecholamines
-
A family of catechol-containing molecules, including adrenaline, noradrenaline and dopamine, which are produced by chromaffin cells of the adrenal medulla and are released as neurotransmitters from postganglionic fibres in the sympathetic nervous system.
- Set point
-
The magnitude of a function in physiology and metabolism (for example, mean arterial blood pressure or body temperature) targeted as the output of homeostasis, such that deviations away from this target initiate compensatory reflexes to adjust the output back to that target.
Rights and permissions
About this article
Cite this article
Tracey, K. Reflex control of immunity. Nat Rev Immunol 9, 418–428 (2009). https://doi.org/10.1038/nri2566
Issue Date:
DOI: https://doi.org/10.1038/nri2566
This article is cited by
-
Cholinergic signaling via the α7 nicotinic acetylcholine receptor regulates the migration of monocyte-derived macrophages during acute inflammation
Journal of Neuroinflammation (2024)
-
The brain cytokine orchestra in multiple sclerosis: from neuroinflammation to synaptopathology
Molecular Brain (2024)
-
Preservation of the pulmonary branches of the vagus nerve during three-dimensional thoracoscopic radical resection of lung cancer: a retrospective study
BMC Surgery (2024)
-
Vagus nerve signal has an inhibitory influence on the development of peritoneal metastasis in murine gastric cancer
Scientific Reports (2024)
-
Galantamine ameliorates experimental pancreatitis
Molecular Medicine (2023)