Key Points
-
Originally, the dorsal visual processing stream was proposed as a 'Where' pathway, supporting spatial processing, but later accounts proposed that it is a 'How' pathway subserving primarily non-conscious visually-guided action.
-
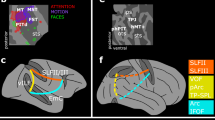
We resolve this debate by showing that at least three pathways emerge from the dorsal stream, supporting three different forms of spatial processing.
-
The parieto–prefrontal pathway connects the posterior parietal with the prefrontal cortex and supports eye movements and spatial working memory.
-
The parieto–premotor pathway connects the posterior parietal with the premotor cortices and supports visually guided action.
-
The parieto–medial temporal pathway is the most complex projection from the posterior parietal cortex. It is a multisynaptic projection emerging from the caudal portion of the inferior parietal lobule and terminating in the parahippocampal cortex and hippocampus, supporting navigation.
-
The intermediate areas along the parieto–medial temporal pathway — the posterior cingulate and retrosplenial cortices — seem to aid in the coordination of allocentric and egocentric spatial representations.
Abstract
The division of cortical visual processing into distinct dorsal and ventral streams is a key framework that has guided visual neuroscience. The characterization of the ventral stream as a 'What' pathway is relatively uncontroversial, but the nature of dorsal stream processing is less clear. Originally proposed as mediating spatial perception ('Where'), more recent accounts suggest it primarily serves non-conscious visually guided action ('How'). Here, we identify three pathways emerging from the dorsal stream that consist of projections to the prefrontal and premotor cortices, and a major projection to the medial temporal lobe that courses both directly and indirectly through the posterior cingulate and retrosplenial cortices. These three pathways support both conscious and non-conscious visuospatial processing, including spatial working memory, visually guided action and navigation, respectively.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Ungerleider, L. G. & Mishkin, M. in Analysis of Visual Behavior (eds Ingle, D. J., Goodale, M. A. & Mansfield, R. J. W.) 549–586 (MIT Press, Cambridge, Massachusetts, 1982).
Mishkin, M., Ungerleider, L. G. & Macko, K. Object vision and spatial vision: two cortical pathways. Trends Neurosci. 6, 414–417 (1983).
Macko, K. A. et al. Mapping the primate visual system with [2–14C]deoxyglucose. Science 218, 394–397 (1982).
Milner, A. D. et al. Perception and action in 'visual form agnosia'. Brain 114, 405–428 (1991).
James, T. W., Culham, J., Humphrey, G. K., Milner, A. D. & Goodale, M. A. Ventral occipital lesions impair object recognition but not object-directed grasping: an fMRI study. Brain 126, 2463–2475 (2003).
Goodale, M. A., Milner, A. D., Jakobson, L. S. & Carey, D. P. A neurological dissociation between perceiving objects and grasping them. Nature 349, 154–156 (1991).
Gentilucci, M. & Rizzolatti, G. in Vision and Action (ed. Goodale, M. A.) 147–162 (Ablex, New York, 1990).
Goodale, M. A. & Milner, A. D. Separate visual pathways for perception and action. Trends Neurosci. 15, 20–25 (1992).
Crick, F. & Koch, C. A framework for consciousness. Nature Neurosci. 6, 119–126 (2003).
Read, J. C., Phillipson, G. P., Serrano-Pedraza, I., Milner, A. D. & Parker, A. J. Stereoscopic vision in the absence of the lateral occipital cortex. PLoS ONE 5, e12608 (2010).
Mishkin, M. in Exploring Brain Functions: Models in Neuroscience (eds Poggio, T. & Glaser, D.) 113–126 (Wiley, 1993).
Byrne, P., Becker, S. & Burgess, N. Remembering the past and imagining the future: a neural model of spatial memory and imagery. Psychol. Rev. 114, 340–375 (2007).
Creem, S. H. & Proffitt, D. R. Defining the cortical visual systems: “what”, “where”, and “how”. Acta Psychol. 107, 43–68 (2001).
Vann, S. D., Aggleton, J. P. & Maguire, E. A. What does the retrosplenial cortex do? Nature Rev. Neurosci. 10, 792–802 (2009).
Aguirre, G. K. & D'Esposito, M. Topographical disorientation: a synthesis and taxonomy. Brain 122, 1613–1628 (1999). An excellent review of topographical disorientation, a disorder that occurs with damage to the regions along the parieto–medial temporal pathway. It is notable because the particular forms of topographical disorientation that result from damage to these regions provides clues to their function.
Galletti, C. et al. The cortical connections of area V6: an occipito-parietal network processing visual information. Eur. J. Neurosci. 13, 1572–1588 (2001).
Galletti, C., Fattori, P., Gamberini, M. & Kutz, D. F. The cortical visual area V6: brain location and visual topography. Eur. J. Neurosci. 11, 3922–3936 (1999).
Colby, C. L., Gattass, R., Olson, C. R. & Gross, C. G. Topographical organization of cortical afferents to extrastriate visual area PO in the macaque: a dual tracer study. J. Comp. Neurol. 269, 392–413 (1988).
Rozzi, S. et al. Cortical connections of the inferior parietal cortical convexity of the macaque monkey. Cereb. Cortex 16, 1389–1417 (2006). A broad survey of the anatomical connectivity across the IPL, providing evidence for the differential connectivity of rIPL and cIPL and the emergence of the parieto–medial temporal pathway from cIPL.
Blatt, G. J., Andersen, R. A. & Stoner, G. R. Visual receptive field organization and cortico-cortical connections of the lateral intraparietal area (area LIP) in the macaque. J. Comp. Neurol. 299, 421–445 (1990).
Cavada, C. & Goldman-Rakic, P. S. Posterior parietal cortex in rhesus monkey: II. Evidence for segregated corticocortical networks linking sensory and limbic areas with the frontal lobe. J. Comp. Neurol. 287, 422–445 (1989).
Schall, J. D., Morel, A., King, D. J. & Bullier, J. Topography of visual cortex connections with frontal eye field in macaque: convergence and segregation of processing streams. J. Neurosci. 15, 4464–4487 (1995). This provides key anatomical evidence for the connections between the posterior parietal cortex and the prefrontal cortex.
Funahashi, S. Prefrontal cortex and working memory processes. Neuroscience 139, 251–261 (2006).
Courtney, S. M., Petit, L., Maisog, J. M., Ungerleider, L. G. & Haxby, J. V. An area specialized for spatial working memory in human frontal cortex. Science 279, 1347–1351 (1998).
Curtis, C. E. Prefrontal and parietal contributions to spatial working memory. Neuroscience 139, 173–180 (2006).
Matelli, M., Govoni, P., Galletti, C., Kutz, D. F. & Luppino, G. Superior area 6 afferents from the superior parietal lobule in the macaque monkey. J. Comp. Neurol. 402, 327–352 (1998).
Gamberini, M. et al. Cortical connections of the visuomotor parietooccipital area V6Ad of the macaque monkey. J. Comp. Neurol. 513, 622–642 (2009). A detailed recent neuroanatomical tracing study showing the involvement of V6Ad within the parieto–premotor pathway.
Nachev, P., Kennard, C. & Husain, M. Functional role of the supplementary and pre-supplementary motor areas. Nature Rev. Neurosci. 9, 856–869 (2008).
Galletti, C., Battaglini, P. P. & Fattori, P. Functional properties of neurons in the anterior bank of the parieto-occipital sulcus of the macaque monkey. Eur. J. Neurosci. 3, 452–461 (1991).
Galletti, C., Battaglini, P. P. & Fattori, P. Eye position influence on the parieto-occipital area PO (V6) of the macaque monkey. Eur. J. Neurosci. 7, 2486–2501 (1995).
Galletti, C., Fattori, P., Kutz, D. F. & Battaglini, P. P. Arm movement-related neurons in the visual area V6A of the macaque superior parietal lobule. Eur. J. Neurosci. 9, 410–413 (1997).
Duhamel, J. R., Colby, C. L. & Goldberg, M. E. Ventral intraparietal area of the macaque: congruent visual and somatic response properties. J. Neurophysiol. 79, 126–136 (1998).
Colby, C. L. & Duhamel, J. R. Heterogeneity of extrastriate visual areas and multiple parietal areas in the macaque monkey. Neuropsychologia 29, 517–537 (1991).
Fattori, P., Gamberini, M., Kutz, D. F. & Galletti, C. 'Arm-reaching' neurons in the parietal area V6A of the macaque monkey. Eur. J. Neurosci. 13, 2309–2313 (2001).
Fattori, P., Kutz, D. F., Breveglieri, R., Marzocchi, N. & Galletti, C. Spatial tuning of reaching activity in the medial parieto-occipital cortex (area V6A) of macaque monkey. Eur. J. Neurosci. 22, 956–972 (2005).
Fattori, P. et al. Hand orientation during reach-to-grasp movements modulates neuronal activity in the medial posterior parietal area V6A. J. Neurosci. 29, 1928–1936 (2009).
Fattori, P. et al. The dorsomedial pathway is not just for reaching: grasping neurons in the medial parieto-occipital cortex of the macaque monkey. J. Neurosci. 30, 342–349 (2010).
Rockland, K. S. & Van Hoesen, G. W. Some temporal and parietal cortical connections converge in CA1 of the primate hippocampus. Cereb. Cortex 9, 232–237 (1999).
Ding, S. L., Van Hoesen, G. & Rockland, K. S. Inferior parietal lobule projections to the presubiculum and neighboring ventromedial temporal cortical areas. J. Comp. Neurol. 425, 510–530 (2000).
Pandya, D. N. & Seltzer, B. Intrinsic connections and architectonics of posterior parietal cortex in the rhesus monkey. J. Comp. Neurol. 204, 196–210 (1982).
Cavada, C. & Goldman-Rakic, P. S. Posterior parietal cortex in rhesus monkey: I. Parcellation of areas based on distinctive limbic and sensory corticocortical connections. J. Comp. Neurol. 287, 393–421 (1989).
Vogt, B. A. & Pandya, D. N. Cingulate cortex of the rhesus monkey: II. Cortical afferents. J. Comp. Neurol. 262, 271–289 (1987).
Morris, R., Pandya, D. N. & Petrides, M. Fiber system linking the mid-dorsolateral frontal cortex with the retrosplenial/presubicular region in the rhesus monkey. J. Comp. Neurol. 407, 183–192 (1999).
Kobayashi, Y. & Amaral, D. G. Macaque monkey retrosplenial cortex: II. Cortical afferents. J. Comp. Neurol. 466, 48–79 (2003).
Kobayashi, Y. & Amaral, D. G. Macaque monkey retrosplenial cortex: III. Cortical efferents. J. Comp. Neurol. 502, 810–833 (2007).
Kondo, H., Saleem, K. S. & Price, J. L. Differential connections of the perirhinal and parahippocampal cortex with the orbital and medial prefrontal networks in macaque monkeys. J. Comp. Neurol. 493, 479–509 (2005).
O'Mara, S. M., Rolls, E. T., Berthoz, A. & Kesner, R. P. Neurons responding to whole-body motion in the primate hippocampus. J. Neurosci. 14, 6511–6523 (1994).
Robertson, R. G., Rolls, E. T., Georges-Francois, P. & Panzeri, S. Head direction cells in the primate pre-subiculum. Hippocampus 9, 206–219 (1999).
Hassabis, D. et al. Decoding neuronal ensembles in the human hippocampus. Curr. Biol. 19, 546–554 (2009).
Bartsch, T. et al. Focal lesions of human hippocampal CA1 neurons in transient global amnesia impair place memory. Science 328, 1412–1415 (2010).
Margulies, D. S. et al. Precuneus shares intrinsic functional architecture in humans and monkeys. Proc. Natl Acad. Sci. USA 106, 20069–20074 (2009). A broad survey of the functional connectivity of the precuneus. Provides critical converging evidence for the existence of the parieto–medial temporal pathway in humans.
Caminiti, R. et al. Understanding the parietal lobe syndrome from a neurophysiological and evolutionary perspective. Eur. J. Neurosci. 31, 2320–2340 (2010).
Vincent, J. L., Kahn, I., Van Essen, D. C. & Buckner, R. L. Functional connectivity of the macaque posterior parahippocampal cortex. J. Neurophysiol. 103, 793–800 (2010).
Rushworth, M. F., Behrens, T. E. & Johansen-Berg, H. Connection patterns distinguish 3 regions of human parietal cortex. Cereb. Cortex 16, 1418–1430 (2006).
Culham, J. C. & Kanwisher, N. G. Neuroimaging of cognitive functions in human parietal cortex. Curr. Opin. Neurobiol. 11, 157–163 (2001).
Boussaoud, D., Ungerleider, L. G. & Desimone, R. Pathways for motion analysis: cortical connections of the medial superior temporal and fundus of the superior temporal visual areas in the macaque. J. Comp. Neurol. 296, 462–495 (1990).
Phinney, R. E. & Siegel, R. M. Speed selectivity for optic flow in area 7a of the behaving macaque. Cereb. Cortex 10, 413–421 (2000).
Duffy, C. J. MST neurons respond to optic flow and translational movement. J. Neurophysiol. 80, 1816–1827 (1998).
Andersen, R. A., Shenoy, K. V., Snyder, L. H., Bradley, D. C. & Crowell, J. A. The contributions of vestibular signals to the representations of space in the posterior parietal cortex. Ann. NY Acad. Sci. 871, 282–292 (1999).
Georgieva, S., Peeters, R., Kolster, H., Todd, J. T. & Orban, G. A. The processing of three-dimensional shape from disparity in the human brain. J. Neurosci. 29, 727–742 (2009).
Genovesio, A. & Ferraina, S. Integration of retinal disparity and fixation-distance related signals toward an egocentric coding of distance in the posterior parietal cortex of primates. J. Neurophysiol. 91, 2670–2684 (2004).
Orban, G. A., Janssen, P. & Vogels, R. Extracting 3D structure from disparity. Trends Neurosci. 29, 466–473 (2006).
Verdon, V., Schwartz, S., Lovblad, K. O., Hauert, C. A. & Vuilleumier, P. Neuroanatomy of hemispatial neglect and its functional components: a study using voxel-based lesion-symptom mapping. Brain 133, 880–894 (2009).
Medina, J. et al. Neural substrates of visuospatial processing in distinct reference frames: evidence from unilateral spatial neglect. J. Cogn. Neurosci. 21, 2073–2084 (2009).
Hillis, A. E. et al. Anatomy of spatial attention: insights from perfusion imaging and hemispatial neglect in acute stroke. J. Neurosci. 25, 3161–3167 (2005).
Konen, C. S. & Kastner, S. Representation of eye movements and stimulus motion in topographically organized areas of human posterior parietal cortex. J. Neurosci. 28, 8361–8375 (2008).
Rawley, J. B. & Constantinidis, C. Neural correlates of learning and working memory in the primate posterior parietal cortex. Neurobiol. Learn. Mem. 91, 129–138 (2009).
Friedman, H. R. & Goldman-Rakic, P. S. Coactivation of prefrontal cortex and inferior parietal cortex in working memory tasks revealed by 2DG functional mapping in the rhesus monkey. J. Neurosci. 14, 2775–2788 (1994).
Chafee, M. V. & Goldman-Rakic, P. S. Matching patterns of activity in primate prefrontal area 8a and parietal area 7ip neurons during a spatial working memory task. J. Neurophysiol. 79, 2919–2940 (1998).
Chafee, M. V. & Goldman-Rakic, P. S. Inactivation of parietal and prefrontal cortex reveals interdependence of neural activity during memory-guided saccades. J. Neurophysiol. 83, 1550–1566 (2000). This reinforces the functional relevance of the parieto–prefrontal pathway by showing the reciprocal effect of inactivation in the posterior parietal and prefrontal cortices.
Todd, J. J. & Marois, R. Capacity limit of visual short-term memory in human posterior parietal cortex. Nature 428, 751–754 (2004).
Sheremata, S. L., Bettencourt, K. C. & Somers, D. C. Hemispheric asymmetry in visuotopic posterior parietal cortex emerges with visual short-term memory load. J. Neurosci. 30, 12581–12588 (2010).
Xu, Y. & Chun, M. M. Dissociable neural mechanisms supporting visual short-term memory for objects. Nature 440, 91–95 (2006).
van Asselen, M. et al. Object-location memory: a lesion-behavior mapping study in stroke patients. Brain Cogn. 71, 287–294 (2009).
Ravizza, S. M., Behrmann, M. & Fiez, J. A. Right parietal contributions to verbal working memory: spatial or executive? Neuropsychologia 43, 2057–2067 (2005).
Pierrot-Deseilligny, C., Ploner, C. J., Muri, R. M., Gaymard, B. & Rivaud-Pechoux, S. Effects of cortical lesions on saccadic: eye movements in humans. Ann. NY Acad. Sci. 956, 216–229 (2002).
Rafal, R. D. Oculomotor functions of the parietal lobe: effects of chronic lesions in humans. Cortex 42, 730–739 (2006).
Milner, A. D. & Goodale, M. A. Two visual systems re-viewed. Neuropsychologia 46, 774–785 (2008).
Snyder, L. H., Grieve, K. L., Brotchie, P. & Andersen, R. A. Separate body- and world-referenced representations of visual space in parietal cortex. Nature 394, 887–891 (1998).
Sereno, M. I. & Huang, R. S. A human parietal face area contains aligned head-centered visual and tactile maps. Nature Neurosci. 9, 1337–1343 (2006).
Prevosto, V., Graf, W. & Ugolini, G. Cerebellar inputs to intraparietal cortex areas LIP and MIP: functional frameworks for adaptive control of eye movements, reaching, and arm/eye/head movement coordination. Cereb. Cortex 20, 214–228 (2010).
Graziano, M. S., Cooke, D. F. & Taylor, C. S. Coding the location of the arm by sight. Science 290, 1782–1786 (2000).
Makin, T. R., Holmes, N. P. & Zohary, E. Is that near my hand? Multisensory representation of peripersonal space in human intraparietal sulcus. J. Neurosci. 27, 731–740 (2007).
Chen, J., Reitzen, S. D., Kohlenstein, J. B. & Gardner, E. P. Neural representation of hand kinematics during prehension in posterior parietal cortex of the macaque monkey. J. Neurophysiol. 102, 3310–3328 (2009).
Padberg, J. et al. Parallel evolution of cortical areas involved in skilled hand use. J. Neurosci. 27, 10106–10115 (2007).
Blangero, A., Menz, M. M., McNamara, A. & Binkofski, F. Parietal modules for reaching. Neuropsychologia 47, 1500–1507 (2009).
Cavina-Pratesi, C., Ietswaart, M., Humphreys, G. W., Lestou, V. & Milner, A. D. Impaired grasping in a patient with optic ataxia: primary visuomotor deficit or secondary consequence of misreaching? Neuropsychologia 48, 226–234 (2010).
Culham, J. C. & Valyear, K. F. Human parietal cortex in action. Curr. Opin. Neurobiol. 16, 205–212 (2006).
Castiello, U. The neuroscience of grasping. Nature Rev. Neurosci. 6, 726–736 (2005).
Goodale, M. A. et al. Separate neural pathways for the visual analysis of object shape in perception and prehension. Curr. Biol. 4, 604–610 (1994). This provides evidence for the importance of the parieto–premotor pathway in visually-guided action by demonstrating that optic ataxia can result from lesions of the posterior parietal cortex. Also shows a double dissociation with patient D.F., whose perception but not action is impaired by ventral stream lesions.
Ishida, H., Nakajima, K., Inase, M. & Murata, A. Shared mapping of own and others' bodies in visuotactile bimodal area of monkey parietal cortex. J. Cogn. Neurosci. 22, 83–96 (2010).
Evangeliou, M. N., Raos, V., Galletti, C. & Savaki, H. E. Functional imaging of the parietal cortex during action execution and observation. Cereb. Cortex 19, 624–639 (2009).
Gardner, E. P. et al. Neurophysiology of prehension. I. Posterior parietal cortex and object-oriented hand behaviors. J. Neurophysiol. 97, 387–406 (2007).
Clower, D. M., Dum, R. P. & Strick, P. L. Basal ganglia and cerebellar inputs to 'AIP'. Cereb. Cortex 15, 913–920 (2005).
Clower, D. M., West, R. A., Lynch, J. C. & Strick, P. L. The inferior parietal lobule is the target of output from the superior colliculus, hippocampus, and cerebellum. J. Neurosci. 21, 6283–6291 (2001).
Lewis, J. W. & Van Essen, D. C. Corticocortical connections of visual, sensorimotor, and multimodal processing areas in the parietal lobe of the macaque monkey. J. Comp. Neurol. 428, 112–137 (2000).
Rozzi, S., Ferrari, P. F., Bonini, L., Rizzolatti, G. & Fogassi, L. Functional organization of inferior parietal lobule convexity in the macaque monkey: electrophysiological characterization of motor, sensory and mirror responses and their correlation with cytoarchitectonic areas. Eur. J. Neurosci. 28, 1569–1588 (2008). A broad survey of the response properties of neurons across the IPL, elucidating the distribution of various visual and somatosensory response properties. These distributions provide evidence for the shift in function between rIPL and cIPL and in doing so highlight the importance of these large-scale surveys of the response properties of single neurons.
Sakata, H. & Kusunoki, M. Organization of space perception: neural representation of three-dimensional space in the posterior parietal cortex. Curr. Opin. Neurobiol. 2, 170–174 (1992).
Chafee, M. V., Crowe, D. A., Averbeck, B. B. & Georgopoulos, A. P. Neural correlates of spatial judgement during object construction in parietal cortex. Cereb. Cortex 15, 1393–1413 (2005).
Chafee, M. V., Averbeck, B. B. & Crowe, D. A. Representing spatial relationships in posterior parietal cortex: single neurons code object-referenced position. Cereb. Cortex 17, 2914–2932 (2007).
Crowe, D. A., Averbeck, B. B. & Chafee, M. V. Neural ensemble decoding reveals a correlate of viewer- to object-centered spatial transformation in monkey parietal cortex. J. Neurosci. 28, 5218–5228 (2008).
Crowe, D. A., Averbeck, B. B., Chafee, M. V. & Georgopoulos, A. P. Dynamics of parietal neural activity during spatial cognitive processing. Neuron 47, 885–891 (2005).
Crowe, D. A., Chafee, M. V., Averbeck, B. B. & Georgopoulos, A. P. Neural activity in primate parietal area 7a related to spatial analysis of visual mazes. Cereb. Cortex 14, 23–34 (2004).
Gron, G., Wunderlich, A. P., Spitzer, M., Tomczak, R. & Riepe, M. W. Brain activation during human navigation: gender-different neural networks as substrate of performance. Nature Neurosci. 3, 404–408 (2000).
Maguire, E. A. et al. Knowing where and getting there: a human navigation network. Science 280, 921–924 (1998).
Doeller, C. F., Barry, C. & Burgess, N. Evidence for grid cells in a human memory network. Nature 463, 657–661 (2010).
Tsao, D. Y. et al. Stereopsis activates V3A and caudal intraparietal areas in macaques and humans. Neuron 39, 555–568 (2003).
Guariglia, C., Piccardi, L., Iaria, G., Nico, D. & Pizzamiglio, L. Representational neglect and navigation in real space. Neuropsychologia 43, 1138–1143 (2005).
Kase, C. S., Troncoso, J. F., Court, J. E., Tapia, J. F. & Mohr, J. P. Global spatial disorientation. Clinico-pathologic correlations. J. Neurol. Sci. 34, 267–278 (1977).
Stark, M., Coslett, H. B. & Saffran, E. M. Impairment of a egocentric map of locations: implication for perception and action. Cogn. Neuropsychol. 13, 418–524 (1996).
Huerta, M. F. & Kaas, J. H. Supplementary eye field as defined by intracortical microstimulation: connections in macaques. J. Comp. Neurol. 293, 299–330 (1990).
Pierrot-Deseilligny, C., Milea, D. & Muri, R. M. Eye movement control by the cerebral cortex. Curr. Opin. Neurol. 17, 17–25 (2004).
Olson, C. R., Musil, S. Y. & Goldberg, M. E. Single neurons in posterior cingulate cortex of behaving macaque: eye movement signals. J. Neurophysiol. 76, 3285–3300 (1996).
McCoy, A. N. & Platt, M. L. Risk-sensitive neurons in macaque posterior cingulate cortex. Nature Neurosci. 8, 1220–1227 (2005).
McCoy, A. N., Crowley, J. C., Haghighian, G., Dean, H. L. & Platt, M. L. Saccade reward signals in posterior cingulate cortex. Neuron 40, 1031–1040 (2003).
Berman, R. A. et al. Cortical networks subserving pursuit and saccadic eye movements in humans: an FMRI study. Hum. Brain Mapp. 8, 209–225 (1999).
Tanabe, J., Tregellas, J., Miller, D., Ross, R. G. & Freedman, R. Brain activation during smooth-pursuit eye movements. Neuroimage 17, 1315–1324 (2002).
Dean, H. L. & Platt, M. L. Allocentric spatial referencing of neuronal activity in macaque posterior cingulate cortex. J. Neurosci. 26, 1117–1127 (2006).
Vogt, B. A., Finch, D. M. & Olson, C. R. Functional heterogeneity in cingulate cortex: the anterior executive and posterior evaluative regions. Cereb. Cortex 2, 435–443 (1992).
Hopfinger, J. B., Buonocore, M. H. & Mangun, G. R. The neural mechanisms of top-down attentional control. Nature Neurosci. 3, 284–291 (2000).
Mesulam, M. M., Nobre, A. C., Kim, Y. H., Parrish, T. B. & Gitelman, D. R. Heterogeneity of cingulate contributions to spatial attention. Neuroimage 13, 1065–1072 (2001).
Small, D. M. et al. The posterior cingulate and medial prefrontal cortex mediate the anticipatory allocation of spatial attention. Neuroimage 18, 633–641 (2003).
Bledowski, C., Rahm, B. & Rowe, J. B. What “works” in working memory? Separate systems for selection and updating of critical information. J. Neurosci. 29, 13735–13741 (2009).
Sato, N., Sakata, H., Tanaka, Y. L. & Taira, M. Context-dependent place-selective responses of the neurons in the medial parietal region of macaque monkeys. Cereb. Cortex 20, 846–858 (2010).
Sato, N., Sakata, H., Tanaka, Y. L. & Taira, M. Navigation-associated medial parietal neurons in monkeys. Proc. Natl Acad. Sci. USA 103, 17001–17006 (2006).
Kovacs, G., Cziraki, C. & Greenlee, M. W. Neural correlates of stimulus-invariant decisions about motion in depth. Neuroimage 51, 329–335 (2010).
Bird, C. M. & Burgess, N. The hippocampus and memory: insights from spatial processing. Nature Rev. Neurosci. 9, 182–194 (2008).
Aggleton, J. P. Understanding retrosplenial amnesia: insights from animal studies. Neuropsychologia 48, 2328–2338 (2010).
Burgess, N. Spatial cognition and the brain. Ann. NY Acad. Sci. 1124, 77–97 (2008).
Iaria, G., Chen, J. K., Guariglia, C., Ptito, A. & Petrides, M. Retrosplenial and hippocampal brain regions in human navigation: complementary functional contributions to the formation and use of cognitive maps. Eur. J. Neurosci. 25, 890–899 (2007).
Epstein, R. A. Parahippocampal and retrosplenial contributions to human spatial navigation. Trends Cogn. Sci. 12, 388–396 (2008).
Maguire, E. A. The retrosplenial contribution to human navigation: a review of lesion and neuroimaging findings. Scand. J. Psychol. 42, 225–238 (2001).
Takahashi, N., Kawamura, M., Shiota, J., Kasahata, N. & Hirayama, K. Pure topographic disorientation due to right retrosplenial lesion. Neurology 49, 464–469 (1997).
Iaria, G., Bogod, N., Fox, C. J. & Barton, J. J. Developmental topographical disorientation: case one. Neuropsychologia 47, 30–40 (2009).
Ino, T. et al. Directional disorientation following left retrosplenial hemorrhage: a case report with fMRI studies. Cortex 43, 248–254 (2007).
Diekmann, V., Jurgens, R. & Becker, W. Deriving angular displacement from optic flow: a fMRI study. Exp. Brain Res. 195, 101–116 (2009).
Baumann, O. & Mattingley, J. B. Medial parietal cortex encodes perceived heading direction in humans. J. Neurosci. 30, 12897–12901 (2010).
Hashimoto, R., Tanaka, Y. & Nakano, I. Heading disorientation: a new test and a possible underlying mechanism. Eur. Neurol. 63, 87–93 (2010). This study is notable for both the specificity of the lesion (case 1), and the simplicity of the task used to demonstrate that RSC is crucial for updating representations after changes in heading.
Committeri, G. et al. Reference frames for spatial cognition: different brain areas are involved in viewer-, object-, and landmark-centered judgments about object location. J. Cogn. Neurosci. 16, 1517–1535 (2004).
Rosenbaum, R. S., Ziegler, M., Winocur, G., Grady, C. L. & Moscovitch, M. “I have often walked down this street before”: fMRI studies on the hippocampus and other structures during mental navigation of an old environment. Hippocampus 14, 826–835 (2004).
Ghaem, O. et al. Mental navigation along memorized routes activates the hippocampus, precuneus, and insula. Neuroreport 8, 739–744 (1997).
Suzuki, M., Tsukiura, T., Matsue, Y., Yamadori, A. & Fujii, T. Dissociable brain activations during the retrieval of different kinds of spatial context memory. Neuroimage 25, 993–1001 (2005).
Epstein, R. A., Parker, W. E. & Feiler, A. M. Where am I now? Distinct roles for parahippocampal and retrosplenial cortices in place recognition. J. Neurosci. 27, 6141–6149 (2007). This provides evidence for the sensitivity of the retrosplenial complex to different forms of scene processing consistent with its complex connectivity with the posterior parietal cortex, the parahippocampal cortex, and hippocampus. It also contrasts retrosplenial complex response with that of the parahippocampal cortex.
Epstein, R. A. & Higgins, J. S. Differential parahippocampal and retrosplenial involvement in three types of visual scene recognition. Cereb. Cortex 17, 1680–1693 (2007).
Galati, G., Pelle, G., Berthoz, A. & Committeri, G. Multiple reference frames used by the human brain for spatial perception and memory. Exp. Brain Res. 206, 109–120 (2010).
Clark, B. J., Bassett, J. P., Wang, S. S. & Taube, J. S. Impaired head direction cell representation in the anterodorsal thalamus after lesions of the retrosplenial cortex. J. Neurosci. 30, 5289–5302 (2010).
Park, S. & Chun, M. M. Different roles of the parahippocampal place area (PPA) and retrosplenial cortex (RSC) in panoramic scene perception. Neuroimage 47, 1747–1756 (2009).
Park, S., Intraub, H., Yi, D. J., Widders, D. & Chun, M. M. Beyond the edges of a view: boundary extension in human scene-selective visual cortex. Neuron 54, 335–342 (2007).
Gramann, K. et al. Human brain dynamics accompanying use of egocentric and allocentric reference frames during navigation. J. Cogn. Neurosci. 22, 2836–2849 (2009).
Park, S., Chun, M. M. & Johnson, M. K. Refreshing and integrating visual scenes in Sscene-selective cortex. J. Cogn. Neurosci. 22, 2813–2822 (2009).
Wolbers, T. & Buchel, C. Dissociable retrosplenial and hippocampal contributions to successful formation of survey representations. J. Neurosci. 25, 3333–3340 (2005).
Saleem, K. S., Price, J. L. & Hashikawa, T. Cytoarchitectonic and chemoarchitectonic subdivisions of the perirhinal and parahippocampal cortices in macaque monkeys. J. Comp. Neurol. 500, 973–1006 (2007). This provides the crucial neuroanatomical evidence necessary to effectively subdivide and characterize the parahippocampal and perirhinal cortices in different macaque species.
Hecaen, H., Tzortzis, C. & Rondot, P. Loss of topographic memory with learning deficits. Cortex 16, 525–542 (1980).
Landis, T., Cummings, J. L., Benson, D. F. & Palmer, E. P. Loss of topographic familiarity. An environmental agnosia. Arch. Neurol. 43, 132–136 (1986).
Takahashi, N. & Kawamura, M. Pure topographical disorientation-the anatomical basis of landmark agnosia. Cortex 38, 717–725 (2002).
Alvarado, M. C. & Bachevalier, J. Comparison of the effects of damage to the perirhinal and parahippocampal cortex on transverse patterning and location memory in rhesus macaques. J. Neurosci. 25, 1599–1609 (2005).
Malkova, L. & Mishkin, M. One-trial memory for object-place associations after separate lesions of hippocampus and posterior parahippocampal region in the monkey. J. Neurosci. 23, 1956–1965 (2003).
Bachevalier, J. & Nemanic, S. Memory for spatial location and object-place associations are differently processed by the hippocampal formation, parahippocampal areas TH/TF and perirhinal cortex. Hippocampus 18, 64–80 (2008).
Sato, N. & Nakamura, K. Visual response properties of neurons in the parahippocampal cortex of monkeys. J. Neurophysiol. 90, 876–886 (2003).
Barrash, J. A historical review of topographical disorientation and its neuroanatomical correlates. J. Clin. Exp. Neuropsychol. 20, 807–827 (1998).
Barrash, J., Damasio, H., Adolphs, R. & Tranel, D. The neuroanatomical correlates of route learning impairment. Neuropsychologia 38, 820–836 (2000).
Habib, M. & Sirigu, A. Pure topographical disorientation: a definition and anatomical basis. Cortex 23, 73–85 (1987).
Mendez, M. F. & Cherrier, M. M. Agnosia for scenes in topographagnosia. Neuropsychologia 41, 1387–1395 (2003).
Aguirre, G. K., Zarahn, E. & D'Esposito, M. An area within human ventral cortex sensitive to “building” stimuli: evidence and implications. Neuron 21, 373–383 (1998).
Epstein, R. & Kanwisher, N. A cortical representation of the local visual environment. Nature 392, 598–601 (1998).
Burgess, N., Maguire, E. A., Spiers, H. J. & O'Keefe, J. A temporoparietal and prefrontal network for retrieving the spatial context of lifelike events. Neuroimage 14, 439–453 (2001).
Janzen, G. & van Turennout, M. Selective neural representation of objects relevant for navigation. Nature Neurosci. 7, 673–677 (2004).
Maguire, E. A., Frith, C. D., Burgess, N., Donnett, J. G. & O'Keefe, J. Knowing where things are parahippocampal involvement in encoding object locations in virtual large-scale space. J. Cogn. Neurosci. 10, 61–76 (1998).
Aguirre, G. K., Detre, J. A., Alsop, D. C. & D'Esposito, M. The parahippocampus subserves topographical learning in man. Cereb. Cortex 6, 823–829 (1996).
Maguire, E. A. Hippocampal involvement in human topographical memory: evidence from functional imaging. Phil. Trans. R. Soc. Lond. B 352, 1475–1480 (1997).
Buffalo, E. A., Bellgowan, P. S. & Martin, A. Distinct roles for medial temporal lobe structures in memory for objects and their locations. Learn. Mem. 13, 638–643 (2006).
Park, S., Brady, T. F., Greene, M. R. & Oliva, A. Disentangling scene content from spatial boundary: complementary roles for the parahippocampal place area and lateral occipital complex in representing real-world scenes. J. Neurosci. 31, 1333–1340 (2011).
Walther, D. B., Caddigan, E., Fei-Fei, L. & Beck, D. M. Natural scene categories revealed in distributed patterns of activity in the human brain. J. Neurosci. 29, 10573–10581 (2009).
Bar, M., Aminoff, E. & Schacter, D. L. Scenes unseen: the parahippocampal cortex intrinsically subserves contextual associations, not scenes or places per se. J. Neurosci. 28, 8539–8544 (2008).
Rolls, E. T. Neurophysiological and computational analyses of the primate presubiculum, subiculum and related areas. Behav. Brain Res. 174, 289–303 (2006).
Taube, J. S. The head direction signal: origins and sensory-motor integration. Annu. Rev. Neurosci. 30, 181–207 (2007).
Matsumura, N. et al. Spatial- and task-dependent neuronal responses during real and virtual translocation in the monkey hippocampal formation. J. Neurosci. 19, 2381–2393 (1999).
Rolls, E. T. Spatial view cells and the representation of place in the primate hippocampus. Hippocampus 9, 467–480 (1999).
Georges-Francois, P., Rolls, E. T. & Robertson, R. G. Spatial view cells in the primate hippocampus: allocentric view not head direction or eye position or place. Cereb. Cortex 9, 197–212 (1999).
Muller, R. U. & Kubie, J. L. The effects of changes in the environment on the spatial firing of hippocampal complex-spike cells. J. Neurosci. 7, 1951–1968 (1987).
O'Keefe, J. & Burgess, N. Geometric determinants of the place fields of hippocampal neurons. Nature 381, 425–428 (1996).
Cressant, A., Muller, R. U. & Poucet, B. Failure of centrally placed objects to control the firing fields of hippocampal place cells. J. Neurosci. 17, 2531–2542 (1997).
Suthana, N. A., Ekstrom, A. D., Moshirvaziri, S., Knowlton, B. & Bookheimer, S. Y. Human hippocampal CA1 involvement during allocentric encoding of spatial information. J. Neurosci. 29, 10512–10519 (2009).
Kumaran, D. & Maguire, E. A. The human hippocampus: cognitive maps or relational memory? J. Neurosci. 25, 7254–7259 (2005).
Aflalo, T. N. & Graziano, M. S. Organization of the macaque extrastriate visual cortex re-examined using the principle of spatial continuity of function. J. Neurophysiol. 105, 305–320 (2011).
Goldman-Rakic, P. S., Selemon, L. D. & Schwartz, M. L. Dual pathways connecting the dorsolateral prefrontal cortex with the hippocampal formation and parahippocampal cortex in the rhesus monkey. Neuroscience 12, 719–743 (1984).
Webster, M. J., Bachevalier, J. & Ungerleider, L. G. Connections of inferior temporal areas TEO and TE with parietal and frontal cortex in macaque monkeys. Cereb. Cortex 4, 470–483 (1994).
Mishkin, M., Suzuki, W. A., Gadian, D. G. & Vargha-Khadem, F. Hierarchical organization of cognitive memory. Phil. Trans. R. Soc. Lond. B 352, 1461–1467 (1997).
Durand, J. B. et al. Anterior regions of monkey parietal cortex process visual 3D shape. Neuron 55, 493–505 (2007).
Srivastava, S., Orban, G. A., De Maziere, P. A. & Janssen, P. A distinct representation of three-dimensional shape in macaque anterior intraparietal area: fast, metric, and coarse. J. Neurosci. 29, 10613–10626 (2009).
Murata, A., Gallese, V., Luppino, G., Kaseda, M. & Sakata, H. Selectivity for the shape, size, and orientation of objects for grasping in neurons of monkey parietal area AIP. J. Neurophysiol. 83, 2580–2601 (2000).
Cohen, Y. E. & Andersen, R. A. Reaches to sounds encoded in an eye-centered reference frame. Neuron 27, 647–652 (2000).
Phan, M. L., Schendel, K. L., Recanzone, G. H. & Robertson, L. C. Auditory and visual spatial localization deficits following bilateral parietal lobe lesions in a patient with Balint's syndrome. J. Cogn. Neurosci. 12, 583–600 (2000).
Pavani, F., Ladavas, E. & Driver, J. Auditory and multisensory aspects of visuospatial neglect. Trends Cogn. Sci. 7, 407–414 (2003).
di Pellegrino, G., Ladavas, E. & Farne, A. Seeing where your hands are. Nature 388, 730 (1997).
Drowos, D. B., Berryhill, M., Andre, J. M. & Olson, I. R. True memory, false memory, and subjective recollection deficits after focal parietal lobe lesions. Neuropsychology 24, 465–475 (2010).
Berryhill, M. E., Picasso, L., Arnold, R., Drowos, D. & Olson, I. R. Similarities and differences between parietal and frontal patients in autobiographical and constructed experience tasks. Neuropsychologia 48, 1385–1393 (2010).
Vinckier, F. et al. “What” and “where” in word reading: ventral coding of written words revealed by parietal atrophy. J. Cogn. Neurosci. 18, 1998–2012 (2006).
Maravita, A. & Iriki, A. Tools for the body (schema). Trends Cogn. Sci. 8, 79–86 (2004).
Mahon, B. Z. et al. Action-related properties shape object representations in the ventral stream. Neuron 55, 507–520 (2007).
Mahon, B. Z., Schwarzbach, J. & Caramazza, A. The representation of tools in left parietal cortex is independent of visual experience. Psychol. Sci. 21, 764–771 (2010).
Tranel, D., Kemmerer, D., Adolphs, R., Damasio, H. & Damasio, A. R. Neural correlates of conceptual knowledge for actions. Cogn. Neuropsychol. 20, 409–432 (2003).
Martin, A. The representation of object concepts in the brain. Annu. Rev. Psychol. 58, 25–45 (2007).
Acknowledgements
We wish to thank L. Ungerleider, M. Goodale, A. Martin, D. Leopold, M. Behrmann and D. Tsao for their extremely helpful comments.This research was supported by the Intramural Program of the US National Institutes of Health (NIH), National Institute of Mental Health (NIMH).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Glossary
- Retinotopic
-
An organization or map in visual cortex that reflects the spatial organization of visual stimuli as they appear on the retina.
- Egocentric
-
An umbrella term for maps and/or patterns of modulation that can be defined in relation to some point on the observer (for example, head- or eye-centred maps).
- Optic flow
-
The apparent motion of the environment caused by relative motion between the observer and the visual surround. During navigation, it can be a source of information about the observer's movement.
- Neglect
-
A deficit resulting from cortical lesions that causes the observer to ignore part of visual space. This deficit can be egocentric, as in hemispatial neglect (in which one half of the visual field is ignored) or allocentric (for example, when the left side of all objects is ignored).
- Allocentric
-
An umbrella term for maps and/or patterns of modulation that are defined in relation to an object exterior to the observer.
- Somatotopic map
-
A map (or a pattern of neural modulation) based on distance from some body part. For example, a cell might increase its firing with decreasing distance of a stimulus from the face or hand
Rights and permissions
About this article
Cite this article
Kravitz, D., Saleem, K., Baker, C. et al. A new neural framework for visuospatial processing. Nat Rev Neurosci 12, 217–230 (2011). https://doi.org/10.1038/nrn3008
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrn3008
This article is cited by
-
Loss of TDP-43 splicing repression occurs early in the aging population and is associated with Alzheimer’s disease neuropathologic changes and cognitive decline
Acta Neuropathologica (2024)
-
Structural brain network analysis in occipital lobe epilepsy
BMC Neurology (2023)
-
A tripartite view of the posterior cingulate cortex
Nature Reviews Neuroscience (2023)
-
A large-scale fMRI dataset for human action recognition
Scientific Data (2023)
-
Does perceiving require perceptual experience?
Review of Philosophy and Psychology (2023)