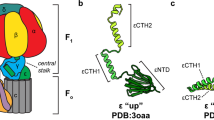
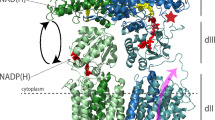
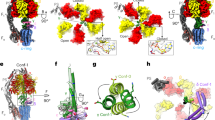
Abstract
Synthesis of ATP from ADP and phosphate, catalyzed by F0F1-ATP synthases, is the most abundant physiological reaction in almost any cell. F0F1-ATP synthases are membrane-bound enzymes that use the energy derived from an electrochemical proton gradient for ATP formation. We incorporated double-labeled F0F1-ATP synthases from Escherichia coli into liposomes and measured single-molecule fluorescence resonance energy transfer (FRET) during ATP synthesis and hydrolysis. The γ subunit rotates stepwise during proton transport–powered ATP synthesis, showing three distinct distances to the b subunits in repeating sequences. The average durations of these steps correspond to catalytic turnover times upon ATP synthesis as well as ATP hydrolysis. The direction of rotation during ATP synthesis is opposite to that of ATP hydrolysis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Mitchell, P. Coupling of phosphorylation to electron and hydrogen transfer by chemi-osmotic type of mechanism. Nature 191, 144–152 (1961).
Boyer, P.D. ATP synthase—past and future. Biochim. Biophys. Acta 1365, 3–9 (1998).
Abrahams, J.P., Leslie, A.G.W., Lutter, R. & Walker, J.E. Structure at 2.8 Å resolution of F1-ATPase from bovine heart mitochondria. Nature 370, 621–628 (1994).
Junge, W., Sabbert, D. & Engelbrecht, S. Rotatory catalysis by F-ATPase: Real-time recording of intersubunit rotation. Ber. Bunsenges. Phys. Chem. 100, 2014–2019 (1996).
Yoshida, M., Muneyuki, E. & Hisabori, T. ATP synthase – a marvelous rotary engine of the cell. Nat. Rev. Mol. Cell. Biol. 2, 669–677 (2001).
Capaldi, R.A. & Aggeler, R. Mechanism of the F1F0-type ATP synthase, a biological rotary motor. Trends Biochem. Sci. 27, 154–160 (2002).
Weber, J. & Senior, A.E. ATP synthesis driven by proton transport in F1F0-ATP synthase. FEBS Lett. 545, 61–70 (2003).
Fillingame, R.H., Angevine, C.M. & Dmitriev, O.Y. Coupling proton movements to c-ring rotation in F1F0 ATP synthase: aqueous access channels and helix rotations at the a-c interface. Biochim. Biophys. Acta 1555, 29–36 (2002).
Noji, H., Yasuda, R., Yoshida, M. & Kinosita, K. Jr. Direct observation of the rotation of F1-ATPase. Nature 386, 299–302 (1997).
Yasuda, R., Noji, H., Yoshida, M., Kinosita, K. & Itoh, H. Resolution of distinct rotational sub-steps by submillisecond kinetic analysis of F1-ATPase. Nature 410, 898–904 (2001).
Yasuda, R. et al. The ATP-waiting conformation of rotating F1-ATPase revealed by single-pair fluorescence resonance energy transfer. Proc. Natl. Acad. Sci. USA 100, 9314–9318 (2003).
Sambongi, Y. et al. Mechanical rotation of the c subunit oligomer in ATP synthase (F0F1): direct observation. Science 286, 1722–1724 (1999).
Junge, W. et al. Inter-subunit rotation and elastic power transmission in F0F1-ATPase. FEBS Lett. 504, 152–160 (2001).
Kaim, G. et al. Coupled rotation within single F0F1 enzyme complexes during ATP synthesis or hydrolysis. FEBS Lett. 525, 156–163 (2002).
Böckmann, R.A. & Grubmüller, H. Nanosecond molecular dynamics simulation of primary mechanical energy transfer steps in F1-ATP synthase. Nat. Struct. Biol. 9, 198–202 (2002).
Ma, J. et al. A dynamical analysis of the rotation mechanism for conformational change in F1-ATPase. Structure 10, 921–930 (2002).
Zhou, Y., Duncan, T.M., Cross, R.L. Subunit rotation in Escherichia coli F0F1-ATP synthase during oxidative phosphorylation. Proc. Natl. Acad. Sci. USA 94, 10583–10587 (1997).
Börsch, M., Diez, M., Zimmermann, B., Reuter, R. & Gräber, P. Stepwise rotation of the γ-subunit of EF0F1-ATP synthase observed by intramolecular single-molecule fluorescence resonance energy transfer. FEBS Lett 527, 147–152 (2002).
Fischer, S. & Gräber, P. Comparison of ΔpH- and Δφ-driven ATP synthesis catalyzed by the H+-ATPases from Escherichia coli or chloroplasts reconstituted into liposomes. FEBS Lett. 457, 327–332 (1999).
Fischer, S., Gräber, P. & Turina, P. The activity of the ATP synthase from Escherichia coli is regulated by the transmembrane proton motive force. J. Biol. Chem. 275, 30157–30162 (2000).
Lötscher, H.R. deJong, C. & Capaldi, R.A. Modification of the F0 portion of the H+-translocating adenosinetriphosphatase complex of Escherichia coli by the water-soluble carbodiimide 1-ethyl-3-[3-(dimethylamino)propyl]carbodiimide and effect on the proton channeling function. Biochemistry 23, 4128–4134 (1983).
Perlin, D.S., Cox, D.N. & Senior, A.E. Integration of F1 and the membrane sector of the proton-ATPase of E coli. J. Biol. Chem. 258, 9793–9800 (1983).
Weiss, S. Measuring conformational dynamics of biomolecules by single molecule fluorescence spectroscopy. Nature Struct. Biol. 7, 724–729 (2000).
Ha, T. Single-molecule fluorescence resonance energy transfer. Methods 25, 78–86 (2001).
Rothwell, P.J. et al. Multi-parameter single-molecule fluorescence spectroscopy reveals heterogeneity of HIV-1 reverse transcriptase: primer/template complexes. Proc. Natl. Acad. Sci. USA 100, 1655–1660 (2003).
Förster, T. Zwischenmolekulare Energiewanderung und Fluoreszenz. Ann. Phys. 2, 55–70 (1948).
Van der Meer, B.W., Cooker, G. & Chen, S.S.-Y. Resonance Energy Transfer: Theory and Data (VCH, New York, 1994).
Häsler, K., Engelbrecht, S. & Junge, W. Three-stepped rotation of subunits Q and O in single molecules of F-ATPase as revealed by polarized, confocal fluorometry. FEBS Lett. 426, 301–304 (1998).
Gogol, E.P., Luecken, U., Bork, T. & Capaldi, R.A. Molecular architecture of Escherichia coli F1 adenosinetriphosphatase. Biochemistry 28, 4709–4716 (1989).
Aggeler, R. & Capaldi, R.A. Cross-linking of the γ subunit of the Escherichia coli ATPase (ECF1) via cysteines introduced by site-directed mutagenesis. J. Biol. Chem. 267, 21355–21359 (1992).
Aggeler, R., Ogilvie, I. & Capaldi, R.A. Rotation of a γ-ε subunit domain in the Escherichia coli F1F0-ATP synthase complex. J. Biol. Chem. 272, 19621–19624 (1997).
Börsch, M. et al. Conformational changes of the H+-ATPase from Escherichia coli upon nucleotide binding detected by single molecule fluorescence. FEBS Lett. 437, 251–254 (1998).
Turina, P. & Capaldi, R.A. ATP hydrolysis-driven structural changes in the γ-subunit of Escherichia coli ATPase monitored by fluorescence from probes bound at introduced cysteine residues. J. Biol. Chem. 269 13465–13471 (1994).
Engelbrecht, S. & Junge, W. ATP synthase: a tentative structural model. FEBS Lett 414, 485–491 (1997).
Rodgers, A. & Wilce, M. Structure of the γ/ε complex of ATP synthase: the camshaft in the rotary motor of life. Nat. Struct. Biol. 7, 1051–1054 (2000).
Wilkens, S., Dunn, S., Chandler, J., Dahlquist, F.W. & Capaldi, R.A. Solution structure of the N-terminal domain of the δ subunit of the E. coli ATP synthase (ECF1F0). Nat. Struct. Biol. 4, 198–201 (1997).
Girvin, M.E., Rastogi, V.K., Abildgaard, F., Markley, J.L. & Fillingame, R.H. Solution structure of the transmembrane H+-transporting subunit c of the F0F1 ATP synthase. Biochemistry. 37, 8817–8824 (1998).
Del Rizzo, P.A., Dunn, S.D., Bi, Y. & Shilton, B.H. The 'second stalk' of Escherichia coli ATP synthase: structure of the isolated dimerization domain. Biochemistry 41, 6875–6884 (2002).
Acknowledgements
This work is dedicated to the memory of K. Süss, who died on 17 March 2002. We thank R.H. Fillingame for his help with the b-mutants, R.A. Capaldi and R. Aggeler for the gift of the γ-mutant, O. Hucke for refinement of the F0F1 model, and M. Antonik and E. Haustein for analytical software. We thank H. Grubmüller, R. Jahn, B.A. Melandri and P. Turina for critical reading the manuscript and helpful discussions and A. Börsch-Haubold for editorial suggestions. C.A.M.S. acknowledges financial support by the Bundesministerium für Bildung und Forschung (BioFuture grant 0311865).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Diez, M., Zimmermann, B., Börsch, M. et al. Proton-powered subunit rotation in single membrane-bound F0F1-ATP synthase. Nat Struct Mol Biol 11, 135–141 (2004). https://doi.org/10.1038/nsmb718
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb718
This article is cited by
-
Machine learning coarse-grained potentials of protein thermodynamics
Nature Communications (2023)
-
Reliability and accuracy of single-molecule FRET studies for characterization of structural dynamics and distances in proteins
Nature Methods (2023)
-
A blind benchmark of analysis tools to infer kinetic rate constants from single-molecule FRET trajectories
Nature Communications (2022)
-
Distance measurements in the F0F1-ATP synthase from E. coli using smFRET and PELDOR spectroscopy
European Biophysics Journal (2020)
-
Temperature-dependent regulation of electron transport and ATP synthesis in chloroplasts in vitro and in silico
Photosynthesis Research (2020)