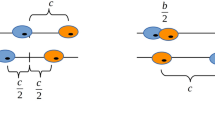
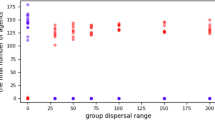
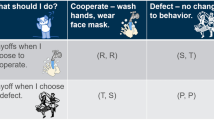
Abstract
Cell cooperation promotes many of the hallmarks of cancer via the secretion of diffusible factors that can affect cancer cells or stromal cells in the tumour microenvironment. This cooperation cannot be explained simply as the collective action of cells for the benefit of the tumour because non-cooperative subclones can constantly invade and free-ride on the diffusible factors produced by the cooperative cells. A full understanding of cooperation among the cells of a tumour requires methods and concepts from evolutionary game theory, which has been used successfully in other areas of biology to understand similar problems but has been underutilized in cancer research. Game theory can provide insights into the stability of cooperation among cells in a tumour and into the design of potentially evolution-proof therapies that disrupt this cooperation.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Jouanneau, J., Moens, G., Bourgeois, Y., Poupon, M. F. & Thiery, J. P. A minority of carcinoma cells producing acidic fibroblast growth factor induces a community effect for tumor progression. Proc. Natl Acad. Sci. USA 91, 286–290 (1994).
Axelrod, R., Axelrod, D. & Pienta, K. J. Evolution of cooperation among tumor cells. Proc. Natl Acad. Sci. USA 103, 13474–13479 (2006).
Archetti, M., Ferraro, D. A. & Christofori, G. Heterogeneity for IGF-II production maintained by public goods dynamics in neuroendocrine pancreatic cancer. Proc. Natl Acad. Sci. USA 112, 1833–1838 (2015).
Cleary, A. S., Leonard, T. L., Gestl, S. A. & Gunther, E. J. Tumour cell heterogeneity maintained by cooperating subclones in Wnt-driven mammary cancers. Nature 508, 113–117 (2014).
Tabassum, D. P. & Polyak, K. Tumorigenesis: it takes a village. Nat. Rev. Cancer 8, 473–483 (2015).
Joyce, J. A. & Pollard, J. W. Microenvironmental regulation of metastasis. Nat. Rev. Cancer 9, 239–252 (2009).
Quail, D. F. & Joyce, J. A. Microenvironmental regulation of tumor progression and metastasis. Nat. Med. 19, 1423–1437 (2013).
Hanahan, D. & Coussens, L. M. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell 21, 309–322 (2012).
MacDougall-Shackleton, S. A. The levels of analysis revisited. Phil. Trans. R. Soc. B 366, 2076–2085 (2011).
Mayr, E. Cause and effect in biology. Science 134, 1501–1506 (1961).
Tinbergen, N. On aims and methods in ethology. Z. Tierpsychol. 20, 410–433 (1963).
Maynard Smith, J. Group selection and kin selection. Nature 201, 1145–1147 (1964).
Williams, G. C. Adaptation and Natural Selection: A Critique of Some Current Evolutionary Thought (Princeton Univ. Press, 1972).
Dawkins, R. The Selfish Gene (Oxford University Press, 1976).
Hardin, G. The tragedy of the commons. Science 162, 1243–1248 (1968).
Nowell, P. C. The clonal evolution of tumor cell populations. Science 194, 23–28 (1976).
Cairns, J. Mutation, selection and the natural history of cancer. Nature 255, 197–200 (1975).
Greaves, M. & Maley, C. C. Clonal evolution in cancer. Nature 481, 306–313 (2012).
Axelrod, R. & Hamilton, W. D. The evolution of cooperation. Science 211, 1390–1396 (1981).
Mesterton-Gibbons, M. & Adams, E. S. The economics of animal cooperation. Science 298, 2146–2147 (2002).
Nowak, M. A. Five rules for the evolution of cooperation. Science 314, 1560–1563 (2006).
West, S. A., Griffin, A. S. & Gardner, A. Evolutionary explanations for cooperation. Curr. Biol. 17, R661–R672 (2007).
Gintis, H. & Bowles, S. A Cooperative Species: Human Reciprocity and Its Evolution (Princeton Univ. Press, 2011).
Tomasello, M. Why We Cooperate (MIT Press, 2009).
Axelrod, R. The Evolution of Cooperation (Basic Books, 1984).
Rasmusen, E. Games and Information: An Introduction To Game Theory (Wiley-Blackwell, 2006).
Osborne, M. An Introduction to Game Theory (Oxford Univ. Press, 2003).
Fudenberg, D. & Tirole, J. Game Theory (MIT Press, 1991).
Myerson, R. Game Theory: Analysis of Conflict (Harvard Univ. Press, 1997).
Maynard Smith, J. Evolution and the Theory of Games (Cambridge Univ. Press, 1982).
McElreath, R. & Boyd, R. Mathematical Models of Social Evolution: A Guide For The Perplexed (Univ. of Chicago Press, 2007).
Dugatkin, L. & Reeve, H. Game Theory and Animal Behaviour (Oxford Univ. Press, 1998).
Hofbauer, J. & Sigmund, K. Evolutionary Games and Population Dynamics (Cambridge Univ. Press, 1998).
Tucker, A. in Reading in Games and Information (ed. Rasmusen, E.) 7–8 (Blackwell Publishers, 2001).
Tomlinson, I. P. Game-theory models of interactions between tumour cells. Eur. J. Cancer 33, 1495–1500 (1997).
Tomlinson, I. P. & Bodmer, W. F. Modelling consequences of interactions between tumour cells. Br. J. Cancer 75, 157–160 (1997).
Rapoport, A. & Chammah, A. M. The game of chicken. Am. Behav. Sci. 10, 10–28 (1966).
Sugden, R. The Economics of Rights, Cooperation and Welfare (B. Blackwell, Oxford, 1986).
Maynard Smith, J. & Price, G. R. The logic of animal conflict. Nature 246, 15–18 (1973).
Bach, L. A., Bentzen, S., Alsner, J. & Christiansen, F. B. An evolutionary-game model of tumour cell interactions, possible relevance to gene therapy. Eur. J. Cancer 37, 2116–2120 (2001).
Bach, L. A., Sumpter, D. J. T., Alsner, J. & Loeschke, V. Spatial evolutionary games of interaction among generic cancer cells. J. Theor. Med. 5, 47–58 (2003).
Basanta, D., Hatzikirou, H. & Deutsch, A. Studying the emergence of invasiveness in tumours using game theory. Eur. Phys. J. 63, 393–397 (2008).
Basanta, D., Simon, M., Hatzikirou, H. & Deutsch, A. Evolutionary game theory elucidates the role of glycolysis in glioma progression and invasion. Cell Prolif. 41, 980–987 (2008).
Basanta, D., Scott, J. G., Rockne, R., Swanson, K. R. & Anderson, A. R. The role of IDH1 mutated tumour cells in secondary glioblastomas, an evolutionary game theoretical view. Phys. Biol. 8, 015016 (2011).
Basanta, D. et al. Investigating prostate cancer tumour-stroma interactions, clinical and biological insights from an evolutionary game. Br. J. Cancer 106, 174–181 (2012).
Dingli, D., Chalub, F. A., Santos, F. C. & Pacheco, J. Cancer phenotype as the outcome of an evolutionary game between normal and malignant cells. Br. J. Cancer 101, 1130–1136 (2009).
Gerstung, M., Nakhoul, H. & Beerenwinkel, N. Evolutionary games with affine fitness functions, applications to cancer. Dyn. Games Appl. 1, 370–385 (2011).
You, L. et al. Spatial versus non-spatial eco-evolutionary dynamics in a tumor growth model. J. Theor. Biol. 435, 78–97 (2017).
Zhang, J. S., Cunningham, J. J., Brown, J. S. & Gatenby, R. A. Integrating evolutionary dynamics into treatment of metastatic castrate-resistant prostate cancer. Nat. Commun. 8, 1816 (2017).
Archetti, M. Dynamics of growth factor production in monolayers of cancer cells. Evol. Appl. 6, 1146–1159 (2013).
Archetti, M. Cooperation among cancer cells as public goods games on Voronoi networks. J. Theor. Biol. 396, 191–203 (2016).
Archetti, M. Stable heterogeneity for the production of diffusible factors in cell populations. PLOS ONE 9, e108526 (2014).
Archetti, M. Evolutionary game theory of growth factor production, implications for tumor heterogeneity and resistance to therapies. Br. J. Cancer 109, 1056–1062 (2013).
Yang, J., Zhao, T. J., Yuan, C. Q., Xie, J. H. & Hao, F. F. A nonlinear competitive model of the prostate tumor growth under intermittent androgen suppression. J. Theor. Biol. 404, 66–72 (2016).
Kianercy, A., Veltri, R. & Pienta, K. J. Critical transitions in a game theoretic model of tumour metabolism. Interface Focus 4, 20140014 (2014).
Kaznatcheev, A., Vander Velde, R., Scott, J. G. & Basanta, D. Cancer treatment scheduling and dynamic heterogeneity in social dilemmas of tumour acidity and vasculature. Br. J. Cancer. 116, 785–792 (2017).
Archetti, M. Evolutionary dynamics of the Warburg effect, glycolysis as a collective action problem among cancer cells. J. Theor. Biol. 341, 1–8 (2014).
Archetti, M. Heterogeneity and proliferation of invasive cancer subclones in game theory models of the Warburg effect. Cell Prolif. 482, 259–269 (2015).
Cirri, P. & Chiarugi, P. Cancer associated fibroblasts: the dark side of the coin. Am. J. Cancer Res. 1, 482–497 (2011).
Sartakhti, J. S., Manshaei, M. H. & Sadeghi, M. MMP-TIMP interactions in cancer invasion: an evolutionary game-theoretical framework. J. Theor. Biol. 412, 17–26 (2017).
Sartakhti, J. S., Manshaei, M. H. & Archetti, M. Game theory of tumor–stroma interactions in multiple myeloma: effect of nonlinear benefits. Games 9, 32 (2018).
Sartakhti, J. S., Manshaei, M. H., Bateni, S. & Archetti, M. Evolutionary dynamics of tumor-stroma interactions in multiple myeloma. PLOS ONE 11, e0168856 (2016).
Kaiser Wilhelm Institut für Biologie. Über Den Stoffwechsel Der Tumoren: The Metabolism of Tumours (ed. Warburg, O.) (Constable, London, 1930).
Nakajima, E. C. & Van Houten, B. Metabolic symbiosis in cancer: refocusing the Warburg lens. Mol. Carcinog. 52, 329–337 (2013).
Cairns, R. A., Harris, I. S. & Mak, T. W. Regulation of cancer cell metabolism. Nat. Rev. Cancer. 11, 85–95 (2011).
Gatenby, R. & Gillies, R. J. Why do cancers have high aerobic glycolysis? Nat. Rev. Cancer 4, 891–899 (2004).
Pavlides, S. et al. The reverse Warburg effect: aerobic glycolysis in cancer associated fibroblasts and the tumor stroma. Cell Cycle 8, 3984–4001 (2009).
Bonuccelli, G. et al. Ketones and lactate “fuel” tumor growth and metastasis: evidence that epithelial cancer cells use oxidative mitochondrial metabolism. Cell Cycle 9, 3506–3514 (2010).
Xing, Y., Zhao, S., Zhou, B. P. & Mi, J. Metabolic reprogramming of the tumour microenvironment. FEBS J. 282, 3892–3898 (2015).
Nieman, K. M. et al. Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nat. Med. 17, 1498–1503 (2011).
Loo, J. M. et al. Extracellular metabolic energetics can promote cancer progression. Cell 160, 393–406 (2015).
Webber, J., Yeung, V. & Clayton, A. Extracellular vesicles as modulators of the cancer microenvironment. Semin. Cell Dev. Biol. 40, 27–34 (2015).
Fong, M. Y. et al. Breast-cancer-secreted miR-122 reprograms glucose metabolism in premetastatic niche to promote metastasis. Nat. Cell Biol. 17, 183–194 (2015).
Archetti, M. et al. Economic game theory for mutualism and cooperation. Ecol. Lett. 14, 1300–1312 (2011).
Archetti, M. & Scheuring, I. Review: game theory of public goods in one-shot social dilemmas without assortment. J. Theor. Biol. 299, 9–20 (2012).
Aktipis, A. Principles of cooperation across systems: from human sharing to multicellularity and cancer. Evol. Appl. 9, 17–36 (2016).
Archetti, M. The volunteer’s dilemma and the optimal size of a social group. J. Theor. Biol. 261, 475–480 (2009).
Archetti, M. Cooperation as a volunteer’s dilemma and the strategy of conflict in public goods games. J. Evol. Biol. 22, 2192–2200 (2009).
Moreno, E. Is cell competition relevant to cancer? Nat. Rev. Cancer 8, 141–147 (2008).
Merino, M. M., Levayer, R. & Moreno, E. Survival of the fittest: essential roles of cell competition in development, aging, and cancer. Trends Cell Biol. 26, 776–788 (2016).
Peter, M. E. et al. The role of CD95 and CD95 ligand in cancer. Cell Death Differ. 22, 549–559 (2015).
Shubik, M. Readings in Game Theory and Political Behavior 43–46 (Doubleday, 1954).
Archetti, M. Survival of the weakest in N-person duels and the maintenance of variation under constant selection. Evolution 66, 637–650 (2012).
Fox, J. & Guyer, M. Public choice and cooperation in N-person prisoner’s dilemma. J. Conflict Resolut. 22, 469–481 (1978).
Hamburger, H. N-person prisoner’s dilemma. J. Math. Sociol. 3, 27–48 (1973).
Grafen, A. & Archetti, M. Natural selection of altruism in inelastic homogeneous viscous populations. J. Theor. Biol. 252, 694–710 (2008).
Cornish-Bowden, A. Fundamentals of Enzyme Kinetics (Wiley, 2012).
Archetti, M. & Scheuring, I. Coexistence of cooperation and defection in public goods games. Evolution 65, 1140–1148 (2011).
Archetti, M. How to analyze models of nonlinear public goods. Games 9, 17 (2018).
de Groot, A. E., Roy, S., Brown, J. S., Pienta, K. J. & Amend, S. R. Revisiting seed and soil: examining the primary tumor and cancer cell foraging in metastasis. Mol. Cancer Res. 15, 361–370 (2017).
Nagy, J. D. Competition and natural selection in a mathematical model of cancer. Bull. Math. Biol. 66, 663–687 (2004).
Archetti, M. & Scheuring, I. Trading public goods stabilizes interspecific mutualism. J. Theor. Biol. 318, 58–67 (2013).
Valkenburg, K. C., de Groot, A. E. & Pienta, K. J. Targeting the tumour stroma to improve cancer therapy. Nat. Rev. Clin. Oncol. 15, 366–381 (2018).
Aktipis, C. A., Kwan, V. S. Y., Johnson, K. A., Neuberg, S. L. & Maley, C. C. Overlooking evolution: a systematic analysis of cancer relapse and therapeutic resistance research. PLOS ONE 6, e26100 (2011).
Myerson, R. B. in The New Palgrave Dictionary of Economics 2nd edn (eds Durlauf, S. N. & Blume, L. E.) 533–542 (Palgrave Macmillan UK, 2008).
Gillies, R. J., Verduzco, D. & Gatenby, R. A. Evolutionary dynamics of carcinogenesis and why targeted therapy does not work. Nat. Rev. Cancer. 12, 487–493 (2012).
Gatenby, R. A. A change of strategy in the war on cancer. Nature 459, 508–509 (2009).
Gatenby, R. A., Silva, A. S., Gillies, R. J. & Frieden, B. R. Adaptive therapy. Cancer Res. 69, 4894–4903 (2009).
Enriquez-Navas, P. M. et al. Exploiting evolutionary principles to prolong tumor control in preclinical models of breast cancer. Sci. Transl Med. 8, 327ra24 (2016).
Read, A. F., Day, T. & Huijben, S. The evolution of drug resistance and the curious orthodoxy of aggressive chemotherapy. Proc. Natl Acad. Sci. USA 108, 10871–10877 (2011).
Day, T., Huijben, V. & Read, A. F. Is selection relevant in the evolutionary emergence of drug resistance? Trends Microbiol. 23, 126–133 (2015).
Day, T. & Read, A. F. Does high-dose antimicrobial chemotherapy prevent the evolution of resistance? PLOS Comput. Biol. 12, e1004689 (2016).
Martin, R., Fisher, M., Minchin, R. & Teo, K. Low-intensity combination chemotherapy maximizes host survival time for tumors containing drug-resistant cells. Math. Biosci. 110, 221–152 (1992).
Hansen, E., Woods, R. J. & Read, A. F. How to use a chemotherapeutic agent when resistance to it threatens the patient. PLOS Biol. 15, e2001110 (2017).
Merlo, L. M. F., Pepper, J. W., Reid, B. J. & Maley, C. C. Cancer as an evolutionary and ecological process. Nat. Rev. Cancer 6, 924–935 (2006).
Silva, A. S. & Gatenby, R. A. A theoretical quantitative model for evolution of cancer chemotherapy resistance. Biol. Direct. 5, 25 (2010).
Gatenby, R. A., Gillies, R. J. & Brown, J. S. The evolutionary dynamics of cancer prevention. Nat. Rev. Cancer 10, 526–527 (2010).
Basanta, D., Gatenby, R. A. & Anderson, A. R. An exploiting evolution to treat drug resistance: combination therapy and the double bind. Mol. Pharm. 9, 914–921 (2012).
Aktipis, C. A. & Nesse, R. M. Evolutionary foundations for cancer biology. Evol. Appl. 6, 144–159 (2013).
André, J. B. & Godelle, B. Multicellular organization in bacteria as a target for drug therapy. Ecol. Lett. 8, 800–810 (2005).
Pepper, J. W. Drugs that target pathogen public goods are robust against evolved drug resistance. Evol. Appl. 5, 757–761 (2012).
Jansen, G., Gatenby, R. & Aktipis, C. A. Opinion: control versus eradication: applying infectious disease treatment strategies to cancer. Proc. Natl Acad. Sci. USA 112, 937–938 (2015).
Archetti, M. Evolutionarily stable anti-cancer therapies by autologous cell defection. Evol. Med. Public Health 1, 161–172 (2013).
Loberg, R. D., Bradley, D. A., Tomlins, S. A., Chinnaiyan, A. M. & Pienta, K. J. The lethal phenotype of cancer: the molecular basis of death due to malignancy. CA Cancer J. Clin. 57, 225–241 (2007).
Maynard Smith, J. & Szathmáry, E. The Major Transitions in Evolution (Oxford Univ. Press, 1995).
Acknowledgements
This work was supported by P01-CA093900, U01-CA196390 and U54-CA210173 and the Prostate Cancer Foundation to K.J.P. and the 7th European Community Framework Program grant agreement No 627816 to M.A.
Reviewer information
Nature Reviews Cancer thanks A. Aktipis, D. Basanta and F. Fu for their contribution to the peer review of this work.
Author information
Authors and Affiliations
Contributions
Both authors researched data for the article, substantially contributed to the discussion of content, and wrote, reviewed and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Clonal selection
-
Natural selection (the preferential survival of the fitter phenotypes) within populations of cells.
- Cooperators
-
Players that pay a cost to produce a benefit for their opponents or contribute to a public good (for example, a growth factor producer).
- Defectors
-
Players that do not produce a benefit for their opponents or do not contribute to a public good (for example, a growth factor non-producer).
- Equilibrium
-
An evolutionarily stable state to which a population converges over time.
- Frequency-dependent
-
A type of natural (clonal) selection in which fitness depends on the frequency of other phenotypes in the population.
- Games
-
The formal description of strategic interactions; they include the definitions of the players, strategies and payoffs.
- Linear effects
-
The effects of cooperation on fitness when the sum of the contributions is additive (each contribution produces the same increment in benefit).
- Multiplayer games
-
Games with multiple players (which can be made of multiple pairwise interactions or a single public goods game).
- Nonlinear effects
-
The effects of cooperation on fitness when the sum of the contributions is not additive but has increasing and/or diminishing returns.
- Optimization
-
The choice of the best set of actions to maximize a payoff function.
- Pairwise game
-
A game with only two players.
- Payoff
-
The reward from the outcome of the interaction (in biology, this is evolutionary fitness).
- Players
-
The individuals (or cells or other entities) that adopt strategies and obtain payoffs.
- Public goods games
-
Multiplayer games in which the payoff depends on the collective decision of multiple players rather than their pairwise interactions.
- Strategy
-
The decision or type adopted by a player (in biology, this is phenotype).
- Warburg effect
-
The switch from aerobic energy production through oxidative phosphorylation to anaerobic energy production through glycolysis.
Rights and permissions
About this article
Cite this article
Archetti, M., Pienta, K.J. Cooperation among cancer cells: applying game theory to cancer. Nat Rev Cancer 19, 110–117 (2019). https://doi.org/10.1038/s41568-018-0083-7
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41568-018-0083-7
This article is cited by
-
A rare case of TFEB/6p21/VEGFA-amplified renal cell carcinoma diagnosed by whole-exome sequencing: clinicopathological and genetic feature report and literature review
Diagnostic Pathology (2024)
-
Game-theoretical description of the go-or-grow dichotomy in tumor development for various settings and parameter constellations
Scientific Reports (2023)
-
Cell facilitation promotes growth and survival under drug pressure in breast cancer
Nature Communications (2023)
-
Evolutionary Games and Applications: Fifty Years of ‘The Logic of Animal Conflict’
Dynamic Games and Applications (2023)
-
Stochastic Fluctuations Drive Non-genetic Evolution of Proliferation in Clonal Cancer Cell Populations
Bulletin of Mathematical Biology (2023)