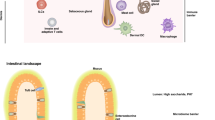
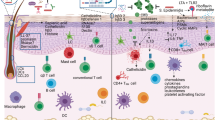
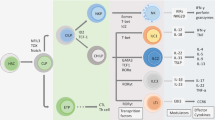
Abstract
The skin is the outermost organ of the body and is continuously exposed to external pathogens. Upon inflammation, various immune cells pass through, reside in or are recruited to the skin to orchestrate diverse cutaneous immune responses. To achieve this, immune cells interact with each other and even communicate with non-immune cells, including peripheral nerves and the microbiota. Immunologically important anatomical sites, such as skin appendages (for example, hair follicles and sweat glands) or postcapillary venules, act as special portal sites for immune cells and for establishing tertiary lymphoid structures, including inducible skin-associated lymphoid tissue. Here, we provide an overview of the key findings and concepts of cutaneous immunity in association with skin anatomy and discuss how cutaneous immune cells fine-tune physiological responses in the skin.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Hoeffel, G. et al. Adult Langerhans cells derive predominantly from embryonic fetal liver monocytes with a minor contribution of yolk sac–derived macrophages. J. Exp. Med. 209, 1167–1181 (2012).
Ginhoux, F. et al. Langerhans cells arise from monocytes in vivo. Nat. Immunol. 7, 265–273 (2006).
Gebhardt, T. et al. Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nat. Immunol. 10, 524–530 (2009).
Masopust, D. et al. Dynamic T cell migration program provides resident memory within intestinal epithelium. J. Exp. Med. 207, 553–564 (2010).
Wakim, L. M., Woodward-Davis, A. & Bevan, M. J. Memory T cells persisting within the brain after local infection show functional adaptations to their tissue of residence. Proc. Natl Acad. Sci. USA 107, 17872–17879 (2010).
Mackay, L. K. et al. Long-lived epithelial immunity by tissue-resident memory T (TRM) cells in the absence of persisting local antigen presentation. Proc. Natl Acad. Sci. USA 109, 7037–7042 (2012).
Wu, T. et al. Lung-resident memory CD8 T cells (TRM) are indispensable for optimal cross-protection against pulmonary virus infection. J. Leukoc. Biol. 95, 215–224 (2014).
Matsui, T. & Amagai, M. Dissecting the formation, structure and barrier function of the stratum corneum. Int. Immunol. 27, 269–280 (2015).
Egawa, G. & Kabashima, K. Multifactorial skin barrier deficiency and atopic dermatitis: essential topics to prevent the atopic march. J. Allergy Clin. Immunol. 138, 350–358.e351 (2016).
Belkaid, Y. & Tamoutounour, S. The influence of skin microorganisms on cutaneous immunity. Nat. Rev. Immunol. 16, 353–366 (2016).
Byrd, A. L., Belkaid, Y. & Segre, J. A. The human skin microbiome. Nat. Rev. Microbiol. 16, 143–155 (2018).
Tong, P. L. et al. The skin immune atlas: three-dimensional analysis of cutaneous leukocyte subsets by multiphoton microscopy. J. Invest. Dermatol. 135, 84–93 (2015).
Lämmermann, T. et al. Neutrophil swarms require LTB4 and integrins at sites of cell death in vivo. Nature 498, 371–375 (2013).
Jain, R., Tikoo, S., Egawa, G. & Weninger, W. in Encyclopedia of Immunobiology Vol. 3 (ed. Ratcliffe, M.) 493–504 (Elsevier, 2016).
Tamoutounour, S. et al. Origins and functional specialization of macrophages and of conventional and monocyte-derived dendritic cells in mouse skin. Immunity 39, 925–938 (2013).
Wolf, K., Müller, R., Borgmann, S., Bröcker, E.-B. & Friedl, P. Amoeboid shape change and contact guidance: T-lymphocyte crawling through fibrillar collagen is independent of matrix remodeling by MMPs and other proteases. Blood 102, 3262–3269 (2003).
Tomura, M. et al. Activated regulatory T cells are the major T cell type emigrating from the skin during a cutaneous immune response in mice. J. Clin. Invest. 120, 883–893 (2010).
Kashem, S. W. et al. Nociceptive sensory fibers drive interleukin-23 production from CD301b+ dermal dendritic cells and drive protective cutaneous immunity. Immunity 43, 515–526 (2015).
Riol-Blanco, L. et al. Nociceptive sensory neurons drive interleukin-23-mediated psoriasiform skin inflammation. Nature 510, 157–161 (2014).
Natsuaki, Y. et al. Perivascular leukocyte clusters are essential for efficient activation of effector T cells in the skin. Nat. Immunol. 15, 1064–1069 (2014). This study shows that perivascular leukocyte clusters including macrophages and DCs are essential structures for effector T cell activation in the skin and proposes the concept of iSALT.
Carmi-Levy, I., Homey, B. & Soumelis, V. A modular view of cytokine networks in atopic dermatitis. Clin. Rev. Allergy Immunol. 41, 245–253 (2011).
Nestle, F. O., Di Meglio, P., Qin, J.-Z. & Nickoloff, B. J. Skin immune sentinels in health and disease. Nat. Rev. Immunol. 9, 679–691 (2009).
Nagao, K. et al. Stress-induced production of chemokines by hair follicles regulates the trafficking of dendritic cells in skin. Nat. Immunol. 13, 744–752 (2012). This is the first study to show the importance of hair follicles for immune cell trafficking from dermis to the epidermis.
Wollenberg, A., Kraft, S., Hanau, D. & Bieber, T. Immunomorphological and ultrastructural characterization of Langerhans cells and a novel, inflammatory dendritic epidermal cell (IDEC) population in lesional skin of atopic eczema. J. Invest. Dermatol. 106, 446–453 (1996).
Liu, Z. et al. Visualization of T cell-regulated monocyte clusters mediating keratinocyte death in acquired cutaneous immunity. J. Invest. Dermatol. 138, 1328–1337 (2018). This study demonstrates that monocytes cluster around hair follicles after hapten painting to the skin.
Paus, R. et al. The hair follicle and immune privilege. J. Investig. Dermatol. Symp. Proc. 8, 188–194 (2003).
Kang, H. et al. Hair follicles from alopecia areata patients exhibit alterations in immune privilege-associated gene expression in advance of hair loss. J. Invest. Dermatol. 130, 2677–2680 (2010).
Adachi, T. et al. Hair follicle–derived IL-7 and IL-15 mediate skin-resident memory T cell homeostasis and lymphoma. Nat. Med. 21, 1272–1279 (2015).
Collins, N. et al. Skin CD4+ memory T cells exhibit combined cluster-mediated retention and equilibration with the circulation. Nat. Commun. 7, 11514 (2016). This study shows that leukocyte clusters around hair follicles may serve as the structures for memory T cell activation after hapten application or HSV infection.
Ali, N. et al. Regulatory T cells in skin facilitate epithelial stem cell differentiation. Cell 169, 1119–1129.e1111 (2017).
Mattii, M. et al. Sebocytes contribute to skin inflammation by promoting the differentiation of T helper 17 cells. Br. J. Dermatol. 178, 722–730 (2018).
Nakahigashi, K. et al. PGD2 induces eotaxin-3 via PPARγ from sebocytes: a possible pathogenesis of eosinophilic pustular folliculitis. J. Allergy Clin. Immunol. 129, 536–543 (2012).
Lai, Y. et al. Commensal bacteria regulate Toll-like receptor 3–dependent inflammation after skin injury. Nat. Med. 15, 1377–1382 (2009).
Linehan, J. L. et al. Non-classical immunity controls microbiota impact on skin immunity and tissue repair. Cell 172, 784–796 (2018).
Grice, E. A. et al. A diversity profile of the human skin microbiota. Genome Res. 18, 1043–1050 (2008).
Egawa, N., Egawa, K., Griffin, H. & Doorbar, J. Human papillomaviruses; epithelial tropisms, and the development of neoplasia. Viruses 7, 3863–3890 (2015).
Egawa, G. et al. Intravital analysis of vascular permeability in mice using two-photon microscopy. Sci. Rep. 3, 1932 (2013).
Abtin, A. et al. Perivascular macrophages mediate neutrophil recruitment during bacterial skin infection. Nat. Immunol. 15, 45–53 (2014).
Szallasi, A., Cortright, D. N., Blum, C. A. & Eid, S. R. The vanilloid receptor TRPV1: 10 years from channel cloning to antagonist proof-of-concept. Nat. Rev. Drug Discov. 6, 357–372 (2007).
van der Fits, L. et al. Imiquimod-induced psoriasis-like skin inflammation in mice is mediated via the IL-23/IL-17 axis. J. Immunol. 182, 5836–5845 (2009).
Feng, J. et al. Sensory TRP channels contribute differentially to skin inflammation and persistent itch. Nat. Commun. 8, 980 (2017).
Nakamizo, S. & Egawa, G. in Immunology of the Skin (ed. Kabashima, K.) 227–238 (Springer, 2016).
Weisberg, S. P. et al. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Invest. 112, 1796–1808 (2003).
Zhang, L.-J. et al. Dermal adipocytes protect against invasive Staphylococcus aureus skin infection. Science 347, 67–71 (2015).
Egawa, G., Miyachi, Y. & Kabashima, K. Identification of perivascular adipose tissue in the mouse skin using two-photon microscopy. J. Dermatol. Sci. 70, 139–140 (2013).
Gao, Y. J., Lu, C., Su, L. Y., Sharma, A. & Lee, R. Modulation of vascular function by perivascular adipose tissue: the role of endothelium and hydrogen peroxide. Br. J. Pharmacol. 151, 323–331 (2007).
Rajsheker, S. et al. Crosstalk between perivascular adipose tissue and blood vessels. Curr. Opin. Pharmacol. 10, 191–196 (2010).
Kubo, A., Nagao, K., Yokouchi, M., Sasaki, H. & Amagai, M. External antigen uptake by Langerhans cells with reorganization of epidermal tight junction barriers. J. Exp. Med. 206, 2937–2946 (2009).
Dress, R. J., Wong, A. Y. & Ginhoux, F. Homeostatic control of dendritic cell numbers and differentiation. Immunol. Cell Biol. 96, 463–476 (2018).
Guilliams, M. et al. Unsupervised high-dimensional analysis aligns dendritic cells across tissues and species. Immunity 45, 669–684 (2016).
Schlitzer, A., McGovern, N. & Ginhoux, F. Dendritic cells and monocyte derived cells: two complementary and integrated functional systems. Semin. Cell Dev. Biol. 41, 9–22 (2017).
Honda, T. et al. Tuning of antigen sensitivity by T cell receptor-dependent negative feedback controls T cell effector function in inflamed tissues. Immunity 40, 235–247 (2014). This study shows a regulatory mechanism of effector T cell motility and its activation status in the skin.
Krummel, M. F., Bartumeus, F. & Gerard, A. T cell migration, search strategies and mechanisms. Nat. Rev. Immunol. 16, 193–201 (2016).
Egawa, G. et al. In vivo imaging of T cell motility in the elicitation phase of contact hypersensitivity using two-photon microscopy. J. Invest. Dermatol. 131, 977–979 (2011).
Matheu, M. P. et al. Imaging of effector memory T cells during a delayed-type hypersensitivity reaction and suppression by Kv1.3 channel block. Immunity 29, 602–614 (2008).
Dudeck, J. et al. Mast cells acquire MHCII from dendritic cells during skin inflammation. J. Exp. Med. 214, 3791–3811 (2017).
Miyake, K. et al. Trogocytosis of peptide-MHC class II complexes from dendritic cells confers antigen-presenting ability on basophils. Proc. Natl Acad. Sci. USA 114, 1111–1116 (2017).
Bennett, C. L. et al. Langerhans cells regulate cutaneous injury by licensing CD8 effector cells recruited to the skin. Blood 117, 7063–7069 (2011).
Kim, J. H. et al. CD1a on Langerhans cells controls inflammatory skin disease. Nat. Immunol. 17, 1159–1166 (2016). This study shows that LCs play crucial roles in the induction of allergic reactions to urushiol as well as in psoriasis via their expression of CD1a.
Ono, S., Honda, T. & Kabashima, K. Requirement of MHC class I on radioresistant cells for granzyme B expression from CD8+ T cells in murine contact hypersensitivity. J. Dermatol. Sci. 90, 98–101 (2018).
Kish, D. D., Volokh, N., Baldwin, W. M. 3rd & Fairchild, R. L. Hapten application to the skin induces an inflammatory program directing hapten-primed effector CD8 T cell interaction with hapten-presenting endothelial cells. J. Immunol. 186, 2117–2126 (2011).
Gaspari, A. A. & Katz, S. I. Induction and functional characterization of class II MHC (Ia) antigens on murine keratinocytes. J. Immunol. 140, 2956–2963 (1988).
Kim, B. S. et al. Keratinocytes function as accessory cells for presentation of endogenous antigen expressed in the epidermis. J. Invest. Dermatol. 129, 2805–2817 (2009).
Krummel, M. F., Heath, W. R. & Allison, J. Differential coupling of second signals for cytotoxicity and proliferation in CD8+ T cell effectors: amplification of the lytic potential by B7. J. Immunol. 163, 2999–3006 (1999).
Streilein, J. W. Skin-associated lymphoid tissues (SALT): origins and functions. J. Invest. Dermatol. 80 (Suppl.), 12–16 (1983).
Streilein, J. W. Circuits and signals of the skin-associated lymphoid tissues (SALT). J. Invest. Dermatol. 85, S10–S13 (1985).
Sontheimer, R. Perivascular dendritic macrophages as immunobiological constituents of the human dermal microvascular unit. J. Invest. Dermatol. 93, S96–S101 (1989).
Honda, T. & Kabashima, K. Novel concept of iSALT (inducible skin-associated lymphoid tissue) in the elicitation of allergic contact dermatitis. Proc. Jpn. Acad. 92, 20–28 (2016).
Kogame, T. et al. Possible inducible skin-associated lymphoid tissue (iSALT)-like structures with CXCL13+ fibroblast-like cells in secondary syphilis. Br. J. Dermatol. 177, 1737–1739 (2017).
Sawada, Y. et al. Resolvin E1 inhibits dendritic cell migration in the skin and attenuates contact hypersensitivity responses. J. Exp. Med. 212, 1921–1930 (2015).
Kashem, S. W., Haniffa, M. & Kaplan, D. H. Antigen-presenting cells in the skin. Annu. Rev. Immunol. 35, 469–499 (2017).
Okada, T., Takahashi, S., Ishida, A. & Ishigame, H. In vivo multiphoton imaging of immune cell dynamics. Pflugers Arch. 468, 1793–1801 (2016). This study shows the in vivo dynamics of skin DC subsets in iSALT by multiphoton microscopy.
Honda, T. et al. Compensatory role of Langerhans cells and langerin-positive dermal dendritic cells in the sensitization phase of murine contact hypersensitivity. J. Allergy Clin. Immunol. 125, 1154–1156.e1152 (2010).
Zaba, L. C. et al. Amelioration of epidermal hyperplasia by TNF inhibition is associated with reduced Th17 responses. J. Exp. Med. 204, 3183–3194 (2007).
Pitzalis, C., Jones, G. W., Bombardieri, M. & Jones, S. A. Ectopic lymphoid-like structures in infection, cancer and autoimmunity. Nat. Rev. Immunol. 14, 447–462 (2014).
Randall, T. D. Bronchus-associated lymphoid tissue (BALT) structure and function. Adv. Immunol. 107, 187–241 (2010).
Dieu-Nosjean, M. C., Goc, J., Giraldo, N. A., Sautes-Fridman, C. & Fridman, W. H. Tertiary lymphoid structures in cancer and beyond. Trends Immunol. 35, 571–580 (2014).
Colbeck, E. J., Ager, A., Gallimore, A. & Jones, G. W. Tertiary lymphoid structures in cancer: drivers of antitumor immunity, immunosuppression, or bystander sentinels in disease? Front. Immunol. 8, 1830 (2017).
Neyt, K., Perros, F., GeurtsvanKessel, C. H., Hammad, H. & Lambrecht, B. N. Tertiary lymphoid organs in infection and autoimmunity. Trends Immunol. 33, 297–305 (2012).
Iijima, N. & Iwasaki, A. A local macrophage chemokine network sustains protective tissue-resident memory CD4 T cells. Science 346, 93–98 (2014). This study shows that leukocyte clusters in genital mucosa after HSV infection are essential structures where activation of memory T cells is induced for the elimination of HSV.
Lowe, P. M. et al. The endothelium in psoriasis. Br. J. Dermatol. 132, 497–505 (1995).
Mitsui, H. et al. Combined use of laser capture microdissection and cDNA microarray analysis identifies locally expressed disease-related genes in focal regions of psoriasis vulgaris skin lesions. J. Invest. Dermatol. 132, 1615–1626 (2012).
Kim, T. G. et al. Dermal clusters of mature dendritic cells and T cells are associated with the CCL20/CCR6 chemokine system in chronic psoriasis. J. Invest. Dermatol. 134, 1462–1465 (2014).
Martinet, L. et al. High endothelial venules (HEVs) in human melanoma lesions: major gateways for tumor-infiltrating lymphocytes. Oncoimmunology 1, 829–839 (2012).
Ladanyi, A. et al. Density of DC-LAMP+ mature dendritic cells in combination with activated T lymphocytes infiltrating primary cutaneous melanoma is a strong independent prognostic factor. Cancer Immunol. Immunother. 56, 1459–1469 (2007).
Arps, D. P. & Patel, R. M. Lupus profundus (panniculitis): a potential mimic of subcutaneous panniculitis-like T cell lymphoma. Arch. Pathol. Lab. Med. 137, 1211–1215 (2013).
Kung, I. T., Gibson, J. B. & Bannatyne, P. M. Kimura’s disease: a clinico-pathological study of 21 cases and its distinction from angiolymphoid hyperplasia with eosinophilia. Pathology 16, 39–44 (1984).
Murata, T. et al. Transient elevation of cytoplasmic calcium ion concentration at a single cell level precedes morphological changes of epidermal keratinocytes during cornification. Sci. Rep. 8, 6610 (2018).
Honda, T., Egawa, G., Grabbe, S. & Kabashima, K. Update of immune events in the murine contact hypersensitivity model: toward the understanding of allergic contact dermatitis. J. Invest. Dermatol. 133, 303–315 (2013).
Kaplan, D. H., Igyártó, B. Z. & Gaspari, A. A. Early immune events in the induction of allergic contact dermatitis. Nat. Rev. Immunol. 12, 114–124 (2012).
Topalian, S. L. et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med. 366, 2443–2454 (2012).
Boissonnas, A. et al. CD8+ tumor-infiltrating T cells are trapped in the tumor-dendritic cell network. Neoplasia 15, 85–94 (2013).
Engelhardt, J. J. et al. Marginating dendritic cells of the tumor microenvironment cross-present tumor antigens and stably engage tumor-specific T cells. Cancer Cell 21, 402–417 (2012).
Broz, M. L. et al. Dissecting the tumor myeloid compartment reveals rare activating antigen-presenting cells critical for T cell immunity. Cancer Cell 26, 638–652 (2014). This study identifies the cutaneous DC subset responsible for antigen presentation in the skin in melanoma.
Gardner, A. & Ruffell, B. Dendritic cells and cancer immunity. Trends Immunol. 37, 855–865 (2016).
Gordon, S. R. et al. PD-1 expression by tumour-associated macrophages inhibits phagocytosis and tumour immunity. Nature 545, 495–499 (2017).
Noordegraaf, M., Flacher, V., Stoitzner, P. & Clausen, B. E. Functional redundancy of Langerhans cells and Langerin+ dermal dendritic cells in contact hypersensitivity. J. Invest. Dermatol. 130, 2752–2759 (2010).
Wohn, C. et al. Langerinneg conventional dendritic cells produce IL-23 to drive psoriatic plaque formation in mice. Proc. Natl Acad. Sci. USA 110, 10723–10728 (2013).
Leinweber, B., Kerl, H. & Cerroni, L. Histopathologic features of cutaneous herpes virus infections (herpes simplex, herpes varicella/zoster): a broad spectrum of presentations with common pseudolymphomatous aspects. Am. J. Surg. Pathol. 30, 50–58 (2006).
Zhu, J. et al. Persistence of HIV-1 receptor-positive cells after HSV-2 reactivation is a potential mechanism for increased HIV-1 acquisition. Nat. Med. 15, 886–892 (2009).
Kiviat, N. B. et al. Endometrial histopathology in patients with culture-proved upper genital tract infection and laparoscopically diagnosed acute salpingitis. Am. J. Surg. Pathol. 14, 167–175 (1990).
Farkas, L., Beiske, K., Lund-Johansen, F., Brandtzaeg, P. & Jahnsen, F. L. Plasmacytoid dendritic cells (natural interferon- α/β-producing cells) accumulate in cutaneous lupus erythematosus lesions. Am. J. Pathol. 159, 237–243 (2001).
Acknowledgements
This work was supported by grants from the Japan Society for the Promotion of Science KAKENHI (JP15K09766, JP15H05096 (to T.H.) and 263395 (to K.K)), Grants-in-Aid for Scientific Research (15H05790, 15H1155 and 15K15417 to K.K.) and the Japan Agency for Medical Research and Development (AMED) (16ek0410011h0003 and 16he0902003h0002 to K.K.). The authors thank A. Hayday of the King’s College London School of Medicine, London, UK, and E. Epstein Jr of PellePharm for the critical reading of the manuscript.
Reviewer information
Nature Reviews Immunology thanks B. Malissen and the other anonymous reviewer(s) for their contribution to the peer review of this work.
Author information
Authors and Affiliations
Contributions
All authors contributed to the discussion of the content of the article. G.E. and T.H. also contributed to researching data and the writing of the article. K.K. and F.G. also contributed to the review and editing of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Langerin-DTR mice
-
Mice that express diphtheria toxin receptor (DTR) under the control of the Langerin gene promoter. Treatment of these mice with diphtheria toxin leads to the deletion of all Langerin-expressing cells.
- Alopecia areata
-
A patchy hair loss mainly occurring in the scalp. It is believed to be one of the autoimmune diseases.
- Eosinophilic pustular folliculitis
-
A recurrent folliculitis that is often formed in the face. In this condition, many eosinophils are pathologically accumulated around hair follicles.
- Transient receptor potential subfamily V member 1
-
(TRPV1). Also known as capsaicin receptor; TRPV1 is a cation channel member selectively expressed on peripheral sensory neurons that serves as a molecular sensor (nociceptor) for noxious stimuli.
- Trogocytosis
-
Lymphocytes that conjugate to antigen-presenting cells sometimes ‘rob’ the surface molecules and express them on their own surfaces. ‘Trogo’ means ‘gnaw’ in Greek.
- Kimura disease
-
A chronic inflammatory disorder characterized by a painless lymphadenopathy or masses on head and neck regions.
Rights and permissions
About this article
Cite this article
Kabashima, K., Honda, T., Ginhoux, F. et al. The immunological anatomy of the skin. Nat Rev Immunol 19, 19–30 (2019). https://doi.org/10.1038/s41577-018-0084-5
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41577-018-0084-5
This article is cited by
-
Using proteomics to compare the molecular structures of sulfide and permeate-depilated sheepskins
Collagen and Leather (2024)
-
Longitudinal assessment of sweat-based TNF-alpha in inflammatory bowel disease using a wearable device
Scientific Reports (2024)
-
Glyoxal-derived advanced glycation end products (GO-AGEs) with UVB critically induce skin inflammaging: in vitro and in silico approaches
Scientific Reports (2024)
-
Development of tight junction-strengthening compounds using a high-throughput screening system to evaluate cell surface-localized claudin-1 in keratinocytes
Scientific Reports (2024)
-
IL-27-induced PD-L1highSca-1+ innate lymphoid cells suppress contact hypersensitivity in an IL-10-dependent manner
Experimental & Molecular Medicine (2024)