Abstract
There is evidence to suggest that increasing the level of saturation (that is, the number of sp3-hybridized carbon atoms) of small molecules can increase their likelihood of success in the drug discovery pipeline1. Owing to their favourable physical properties, alkylamines have become ubiquitous among pharmaceutical agents, small-molecule biological probes and pre-clinical candidates2. Despite their importance, the synthesis of amines is still dominated by two methods: N-alkylation and carbonyl reductive amination3. Therefore, the increasing demand for saturated polar molecules in drug discovery has continued to drive the development of practical catalytic methods for the synthesis of complex alkylamines4,5,6,7. In particular, processes that transform accessible feedstocks into sp3-rich architectures provide a strategic advantage in the synthesis of complex alkylamines. Here we report a multicomponent, reductive photocatalytic technology that combines readily available dialkylamines, carbonyls and alkenes to build architecturally complex and functionally diverse tertiary alkylamines in a single step. This olefin-hydroaminoalkylation process involves a visible-light-mediated reduction of in-situ-generated iminium ions to selectively furnish previously inaccessible alkyl-substituted α-amino radicals, which subsequently react with alkenes to form C(sp3)–C(sp3) bonds. The operationally straightforward reaction exhibits broad functional-group tolerance, facilitates the synthesis of drug-like amines that are not readily accessible by other methods and is amenable to late-stage functionalization applications, making it of interest in areas such as pharmaceutical and agrochemical research.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data that support the findings of this study are available within the paper and its supplementary information files. Raw data are available from the corresponding author on reasonable request. Materials and methods, experimental procedures, useful information, mechanistic studies, optimization studies, 1H NMR spectra, 13C NMR spectra and mass spectrometry data are available in the Supplementary Materials. Crystallographic data are available free of charge from the Cambridge Crystallographic Data Centre (https://www.ccdc.cam.ac.uk/) under reference number CCDC 1819790.
References
Lovering, F., Bikker, J. & Humblet, C. Escape from flatland: Increasing saturation as an approach to improving clinical success. J. Med. Chem. 52, 6752–6756 (2009).
Blakemore, D. C. et al. Organic synthesis provides opportunities to transform drug discovery. Nat. Chem. 10, 383–394 (2018).
Roughley, S. D. & Jordan, A. M. The medicinal chemist’s toolbox: An analysis of reactions used in the pursuit of drug candidates. J. Med. Chem. 54, 3451–3479 (2011).
Yang, Y., Shi, S.-L., Niu, D., Liu, P. & Buchwald, S. L. Catalytic asymmetric hydroamination of unactivated internal olefins to aliphatic amines. Science 349, 62–66 (2015).
Musacchio, A. J. et al. Catalytic intermolecular hydroaminations of unactivated olefins with secondary alkyl amines. Science 355, 727–730 (2017).
Johnston, C. P., Smith, R. T., Allmendinger, S. & MacMillan, D. W. C. Metallophotoredox-catalysed sp 3 –sp 3 cross-coupling of carboxylic acids with alkyl halides. Nature 536, 322–325 (2016).
Matier, C. D., Schwaben, J., Peters, J. C. & Fu, G. C. Copper-catalyzed alkylation of aliphatic amines induced by visible light. J. Am. Chem. Soc. 139, 17707–17710 (2017).
Mayol-Llinàs, J., Nelson, A., Farnaby, W. & Ayscough, A. Assessing molecular scaffolds for CNS drug discovery. Drug Discov. Today 22, 965–969 (2017).
Lahmy, V., Long, R., Morin, D., Villard, V. & Maurice, T. Mitochondrial protection by the mixed muscarinic/σ1 ligand ANAVEX2–73, a tetrahydrofuran derivative, in Aβ25–35 peptide-injected mice, a nontransgenic Alzheimer’s disease model. Front. Cell. Neurosci. 8, 463 (2015).
Marciano, D. P. et al. The therapeutic potential of nuclear receptor modulators for treatment of metabolic disorders: PPARγ, RORs, Rev-erbs. Cell Metab. 19, 193–208 (2014).
Ruiz-Castillo, P. & Buchwald, S. L. Application of palladium-catalyzed C–N cross-coupling reactions. Chem. Rev. 116, 12564–12649 (2016).
Huang, L., Arndt, M., Gooßen, K., Heydt, H. & Gooßen, L. J. Late transition metal-catalyzed hydroamination and hydroamidation. Chem. Rev. 115, 2596–2697 (2015).
Kalck, P. & Urrutigoïty, M. Tandem hydroaminomethylation reaction to synthesize amines from alkenes. Chem. Rev. 118, 3833–3861 (2018).
Herzon, S. B. & Hartwig, J. F. Hydroaminoalkylation of unactivated olefins with dialkylamines. J. Am. Chem. Soc. 130, 14940–14941 (2008).
Perez, F., Oda, S., Geary, L. M. & Krische, M. J. Ruthenium catalyzed transfer hydrogenation for C–C bond formation: hydrohydroxyalkylation and hydroaminoalkylation via reactant redox pairs. Top. Curr. Chem. 374, 35 (2016).
Renaud, P. & Giraud, L. 1-Amino- and 1-amidoalkyl radicals: generation and stereoselective reactions. Synthesis 1996, 913–926 (1996).
Mariano, P. S. Electron-transfer mechanisms in photochemical transformations of iminium salts. Acc. Chem. Res. 16, 130–137 (1983).
Chu, L., Ohta, C., Zuo, Z. & MacMillan, D. W. C. Carboxylic acids as a traceless activation group for conjugate additions: a three-step synthesis of (±)-pregabalin. J. Am. Chem. Soc. 136, 10886–10889 (2014).
Nakajima, K., Miyake, Y. & Nishibayashi, Y. Synthetic utilization of α-amino radicals and related species in visible light photoredox catalysis. Acc. Chem. Res. 49, 1946–1956 (2016).
Thullen, S. M. & Rovis, T. A mild hydroaminoalkylation of conjugated dienes using a unified cobalt and photoredox catalytic system. J. Am. Chem. Soc. 139, 15504–15508 (2017).
Andrieux, C. P. & Savéant, J. M. Electrodimerization: II. Reduction mechanism of immonium cations. J. Electroanal. Chem. 26, 223–235 (1970).
Lee, K. N. & Ngai, M.-Y. Recent developments in transition-metal photoredox-catalysed reactions of carbonyl derivatives. Chem. Commun. 53, 13093–13112 (2017).
Heinz, C. et al. Ni-catalyzed carbon–carbon bond-forming reductive amination. J. Am. Chem. Soc. 140, 2292–2300 (2018).
Capacci, A. G., Malinowski, J. T., McAlpine, M. J., Kuhne, J. & MacMillan, D. W. C. Direct, enantioselective α-alkylation of aldehydes using simple olefins. Nat. Chem. 9, 1073–1077 (2017).
Dai, X. et al. The coupling of tertiary amines and acrylate derivatives via visible-light photoredox catalysis. J. Org. Chem. 79, 7212–7219 (2014).
Guo, X. & Wenger, O. S. Reductive amination by photoredox catalysis and polarity-matched hydrogen atom transfer. Angew. Chem. Int. Ed. 57, 2469–2473 (2018).
Flamigni, L., Barbieri, A., Sabatini, C., Ventura, B. & Barigelletti, F. Photochemistry and photophysics of coordination compounds: iridium. Top. Curr. Chem. 281, 143–203 (2007).
Yu, P. et al. Enantioselective C–H bond functionalization triggered by radical trifluoromethylation of unactivated alkene. Angew. Chem. Int. Ed. 53, 11890–11894 (2014).
Wayner, D. D. M., Dannenberg, J. J. & Griller, D. Oxidation potentials of α-aminoalkyl radicals: bond dissociation energies for related radical cations. Chem. Phys. Lett. 131, 189–191 (1986).
Simons, F. E. R. Advances in H1-antihistamines. N. Engl. J. Med. 351, 2203–2217 (2004).
Hager, A., Vrielink, N., Hager, D., Lefranc, J. & Trauner, D. Synthetic approaches towards alkaloids bearing α-tertiary amines. Nat. Prod. Rep. 33, 491–522 (2016).
Acknowledgements
We thank A. Bond for X-ray crystallographic analysis, the EPSRC UK National Mass Spectrometry Facility at Swansea University for HRMS analysis, and M. Nappi, J. C. K. Chu and T. Hunt (AstraZeneca) for useful discussion. We acknowledge the Herschel Smith Scholarship Scheme and AstraZeneca for studentships (to A.T. and D.R., respectively) and the Royal Society for a Wolfson Merit Award (M.J.G.).
Author information
Authors and Affiliations
Contributions
M.J.G., A.T. and D.R. designed the experiments. A.T. and D.R. performed and analysed the experiments. M.J.G., A.T. and D.R prepared the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
This file contains Supplementary Materials and Methods, Supplementary Figs. 1–5, Supplementary Tables 1–2, Supplementary References and 1H and 13C NMR Spectral Data.
Supplementary Data
This file contains the crystallographic information.
Rights and permissions
About this article
Cite this article
Trowbridge, A., Reich, D. & Gaunt, M.J. Multicomponent synthesis of tertiary alkylamines by photocatalytic olefin-hydroaminoalkylation. Nature 561, 522–527 (2018). https://doi.org/10.1038/s41586-018-0537-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-018-0537-9
Keywords
This article is cited by
-
Cobalt-catalyzed aminoalkylative carbonylation of alkenes toward direct synthesis of γ-amino acid derivatives and peptides
Nature Communications (2023)
-
Modular assembly of indole alkaloids enabled by multicomponent reaction
Nature Communications (2023)
-
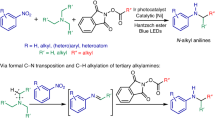
Metallaphotocatalytic synthesis of anilines through tandem C–N transposition and C–H alkylation of alkylamines
Nature Synthesis (2023)
-
Recyclable Copper-Catalyzed Decarboxylative C–C Coupling of the sp3-Hybridized Carbon Atoms of α-Amino Acids
Catalysis Letters (2023)
-
Multicomponent double Mannich alkylamination involving C(sp2)–H and benzylic C(sp3)–H bonds
Nature Communications (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.