Abstract
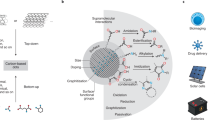
Carbon-based dots (CDs) and their functionalized (nano)composites have recently attracted attention due to their seemingly easy preparation and numerous potential applications, ranging from those in the biomedical field (i.e., imaging and drug delivery) to those in (opto)electronics (i.e., solar cells and LEDs). This protocol details step-by-step procedures for synthesis, purification, functionalization and characterization of nitrogen-doped carbon nanodots (NCNDs), which we have been preparing for the past few years. First, we describe the bottom-up synthesis of NCNDs, starting with the use of molecular precursors (arginine (Arg) and ethylenediamine (EDA)) and making use of microwave-assisted hydrothermal heating. We also provide guidelines for the purification of these materials, through either dialysis or low-pressure size-exclusion chromatography (SEC). Second, we outline post-functionalization procedures for the surface modification of NCNDs, such as alkylation and amidation reactions. Third, we provide instructions for the preparation of NCNDs with different properties, such as color emission, electrochemistry and chirality. Given the fast evolution of preparations and applications of CDs, issues that might arise from artifacts, errors and impurities should be avoided. In this context, the present protocol aims to provide details and guidelines for the synthesis of high-quality nanomaterials with high reproducibility, for various applications. Furthermore, specific needs might require the CDs to be prepared by different synthetic procedures and/or from different molecular precursors, but such CDs can still benefit from the purification and characterization procedures outlined in this protocol. The sample preparation takes various time frames, ranging from 4 to 18 d, depending on the adopted synthesis and purification steps.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
The main data supporting the findings of this study are available within the article and its Supplementary Information files. Additional data are available from the corresponding authors upon request.
References
Whitesides, G. M. & Grzybowski, B. Self-assembly at all scales. Science 295, 2418–2421 (2002).
Gates, B. D. et al. New approaches to nanofabrication: molding, printing, and other techniques. Chem. Rev. 105, 1171–1196 (2005).
Peer, D. et al. Nanocarriers as an emerging platform for cancer therapy. Nat. Nanotechnol. 2, 751–760 (2007).
Thompson, M. et al. Understanding biophysicochemical interactions at the nano–bio interface. Nat. Mater. 8, 543–557 (2009).
Krueger, A. Carbon Materials and Nanotechnology (John Wiley & Sons, 2010).
Albanese, A., Tang, P. S. & Chan, W. C. W. The effect of nanoparticle size, shape, and surface chemistry on biological systems. Annu. Rev. Biomed. Eng. 14, 1–16 (2012).
Pelaz, B. et al. Diverse applications of nanomedicine. ACS Nano 11, 2313–2381 (2017).
Baker, S. N. & Baker, G. A. Luminescent carbon nanodots: emergent nanolights. Angew. Chem. Int. Ed. 49, 6726–6744 (2010).
Georgakilas, V., Perman, J. A., Tucek, J. & Zboril, R. Broad family of carbon nanoallotropes: classification, chemistry, and applications of fullerenes, carbon dots, Nanotubes, graphene, nanodiamonds, and combined superstructures. Chem. Rev. 115, 4744–4822 (2015).
Zheng, X. T., Ananthanarayanan, A., Luo, K. Q. & Chen, P. Glowing graphene quantum dots and carbon dots: properties, syntheses, and biological applications. Small 11, 1620–1636 (2015).
Hutton, G. A. M., Martindale, B. C. M. & Reisner, E. Carbon dots as photosensitisers for solar-driven catalysis. Chem. Soc. Rev. 46, 6111–6123 (2017).
Choi, Y., Choi, Y., Kwon, O.-H. & Kim, B.-S. Carbon dots: bottom-up syntheses, Properties, and light-harvesting applications. Chem. Asian J. 13, 586–598 (2018).
Strauss, V., Roth, A., Sekita, M. & Guldi, D. M. Efficient energy-conversion materials for the future: understanding and tailoring charge-transfer processes in carbon nanostructures. Chem 1, 531–556 (2016).
Antonietti, M. & Oschatz, M. The concept of “noble, heteroatom-doped carbons,” their directed synthesis by electronic band control of carbonization, and applications in catalysis and energy materials. Adv. Mater. 30, 1706836 (2018).
Bhattacharyya, S. et al. Effect of nitrogen atom positioning on the trade-off between emissive and photocatalytic properties of carbon dots. Nat. Commun. 8, 1401 (2017).
Li, F., Yang, D. & Xu, H. Non-metal-heteroatom-doped carbon dots: synthesis and properties. Chem. Eur. J. 25, 1165–1176 (2019).
Kim, D. et al. Graphene quantum dots prevent α-synucleinopathy in Parkinson’s disease. Nat. Nanotechnol. 13, 812–818 (2018).
Green, D. C. et al. Controlling the fluorescence and room-temperature phosphorescence behaviour of carbon nanodots with inorganic crystalline nanocomposites. Nat. Commun. 10, 206 (2019).
Li, Q. et al. Induction of long-lived room temperature phosphorescence of carbon dots by water in hydrogen-bonded matrices. Nat. Commun. 9, 734 (2018).
Xiong, Y., Schneider, J., Ushakova, E. V. & Rogach, A. L. Influence of molecular fluorophores on the research field of chemically synthesized carbon dots. Nano Today 23, 124–139 (2018).
Cailotto, S. et al. Design of carbon dots for metal-free photoredox catalysis. ACS Appl. Mater. Interfaces 10, 40560–40567 (2018).
Yan, Y. et al. Systematic bandgap engineering of graphene quantum dots and applications for photocatalytic water splitting and CO2 reduction. ACS Nano 12, 3523–3532 (2018).
Yuan, F. et al. Engineering triangular carbon quantum dots with unprecedented narrow bandwidth emission for multicolored LEDs. Nat. Commun. 9, 2249 (2018).
Lim, S. Y., Shen, W. & Gao, Z. Carbon quantum dots and their applications. Chem. Soc. Rev. 44, 362–381 (2015).
Zhang, J. & Yu, S.-H. Carbon dots: large-scale synthesis, sensing and bioimaging. Mater. Today 19, 382–393 (2016).
Pham, S. N. et al. Carbon dots: a modular activity to teach fluorescence and nanotechnology at multiple levels. J. Chem. Educ. 94, 1143–1149 (2017).
Schneider, E. M., Bärtsch, A., Stark, W. J. & Grass, R. N. Safe one-pot synthesis of fluorescent carbon quantum dots from lemon juice for a hands-on experience of nanotechnology. J. Chem. Educ. 96, 540–545 (2019).
Cayuela, A., Soriano, M. L., Carrillo-Carrión, C. & Valcárcel, M. Semiconductor and carbon-based fluorescent nanodots: the need for consistency. Chem. Commun. 52, 1311–1326 (2016).
Essner, J. B., Kist, J. A., Polo-Parada, L. & Baker, G. A. Artifacts and errors associated with the ubiquitous presence of fluorescent impurities in carbon nanodots. Chem. Mater. 30, 1878–1887 (2018).
Arcudi, F., Đorđević, L. & Prato, M. Synthesis, separation, and characterization of small and highly fluorescent nitrogen-doped carbon nanodots. Angew. Chem. Int. Ed. 55, 2107–2112 (2016).
Arcudi, F., Đorđević, L. & Prato, M. Design, synthesis, and functionalization strategies of tailored carbon nanodots. Acc. Chem. Res. 52, 2070–2079 (2019).
Ðorđević, L. et al. Design principles of chiral carbon nanodots help convey chirality from molecular to nanoscale level. Nat. Commun. 9, 3442 (2018).
Arcudi, F., Đorđević, L. & Prato, M. Rationally designed carbon nanodots towards pure white-light emission. Angew. Chem. Int. Ed. 56, 4170–4173 (2017).
Rigodanza, F., Đorđević, L., Arcudi, F. & Prato, M. Customizing the electrochemical properties of carbon nanodots by using quinones in bottom-up synthesis. Angew. Chem. Int. Ed. 57, 5062–5067 (2018).
Carrara, S., Arcudi, F., Prato, M. & De Cola, L. Amine-rich nitrogen-doped carbon nanodots as a platform for self-enhancing electrochemiluminescence. Angew. Chem. Int. Ed. 56, 4757–4761 (2017).
Arcudi, F. et al. Porphyrin antennas on carbon nanodots: excited state energy and electron transduction. Angew. Chem. Int. Ed. 56, 12097–12101 (2017).
Cadranel, A. et al. Screening supramolecular interactions between carbon nanodots and porphyrins. J. Am. Chem. Soc. 140, 904–907 (2018).
Dimos, K. et al. Top-down and bottom-up approaches to transparent, flexible and luminescent nitrogen-doped carbon nanodot-clay hybrid films. Nanoscale 9, 10256–10262 (2017).
Rizzo, C. et al. Nitrogen-doped carbon nanodots-ionogels: preparation, characterization, and radical scavenging activity. ACS Nano 12, 1296–1305 (2018).
Gomez, I. J., Arnaiz, B., Cacioppo, M., Arcudi, F. & Prato, M. Nitrogen-doped carbon nanodots for bioimaging and delivery of paclitaxel. J. Mater. Chem. B 6, 5540–5548 (2018).
Sciortino, A. et al. β-C3N4 nanocrystals: carbon dots with extraordinary morphological, structural, and optical homogeneity. Chem. Mater. 30, 1695–1700 (2018).
Xu, X. et al. Electrophoretic analysis and purification of fluorescent single-walled carbon nanotube fragments. J. Am. Chem. Soc. 126, 12736–12737 (2004).
Sun, Y.-P. et al. Quantum-sized carbon dots for bright and colorful photoluminescence. J. Am. Chem. Soc. 128, 7756–7757 (2006).
Zhou, J. et al. An electrochemical avenue to blue luminescent nanocrystals from multiwalled carbon nanotubes (MWCNTs). J. Am. Chem. Soc. 129, 744–745 (2007).
Ye, R. et al. Coal as an abundant source of graphene quantum dots. Nat. Commun. 4, 2943 (2013).
Ming, H. et al. Large scale electrochemical synthesis of high quality carbon nanodots and their photocatalytic property. Dalt. Trans. 41, 9526–9531 (2012).
Jiang, J., He, Y., Li, S. & Cui, H. Amino acids as the source for producing carbon nanodots: microwave assisted one-step synthesis, intrinsic photoluminescence property and intense chemiluminescence enhancement. Chem. Commun. 48, 9634 (2012).
Zhu, S., Zhao, X., Song, Y., Lu, S. & Yang, B. Beyond bottom-up carbon nanodots: citric-acid derived organic molecules. Nano Today 11, 128–132 (2016).
Schneider, J. et al. Molecular fluorescence in citric acid-based carbon dots. J. Phys. Chem. C. 121, 2014–2022 (2017).
Hill, S. & Galan, M. C. Fluorescent carbon dots from mono- and polysaccharides: synthesis, properties and applications. Beilstein J. Org. Chem. 13, 675–693 (2017).
Titirici, M. M. & Antonietti, M. Chemistry and materials options of sustainable carbon materials made by hydrothermal carbonization. Chem. Soc. Rev. 39, 103–116 (2010).
Schwenke, A. M., Hoeppener, S. & Schubert, U. S. Synthesis and modification of carbon nanomaterials utilizing microwave heating. Adv. Mater. 27, 4113–4141 (2015).
Krysmann, M. J., Kelarakis, A., Dallas, P. & Giannelis, E. P. Formation mechanism of carbogenic nanoparticles with dual photoluminescence emission. J. Am. Chem. Soc. 134, 747–750 (2012).
Jiang, K. et al. Red, green, and blue luminescence by carbon dots: Full-color emission tuning and multicolor cellular imaging. Angew. Chem. Int. Ed. 54, 5360–5363 (2015).
Yuan, F. et al. Bright multicolor bandgap fluorescent carbon quantum dots for electroluminescent light-emitting diodes. Adv. Mater. 29, 1604436 (2017).
Ding, H. et al. Solvent-controlled synthesis of highly luminescent carbon dots with a wide color gamut and narrowed emission peak widths. Small 14, 1800612 (2018).
Miao, X. et al. Synthesis of carbon dots with multiple color emission by controlled graphitization and surface functionalization. Adv. Mater. 30, 1704740 (2018).
Li, L. & Dong, T. Photoluminescence tuning in carbon dots: surface passivation or/and functionalization, heteroatom doping. J. Mater. Chem. C. 6, 7944–7970 (2018).
Deng, J. et al. Electrochemical synthesis of carbon nanodots directly from alcohols. Chem. Eur. J. 20, 4993–4999 (2014).
Yao, B., Huang, H., Liu, Y. & Kang, Z. Carbon dots: a small conundrum. Trends Chem. 1, 235–246 (2019).
Ţucureanu, V., Matei, A. & Avram, A. M. FTIR spectroscopy for carbon family study. Crit. Rev. Anal. Chem. 46, 502–520 (2016).
Liu, J. et al. Metal-free efficient photocatalyst for stable visible water splitting via a two-electron pathway. Science 347, 970–974 (2015).
Koutsioukis, A., Akouros, A., Zboril, R. & Georgakilas, V. Solid phase extraction for the purification of violet, blue, green and yellow emitting carbon dots. Nanoscale 10, 11293–11296 (2018).
Zhou, Y. et al. Photoluminescent carbon dots: a mixture of heterogeneous fractions. ChemPhysChem 19, 2589–2597 (2018).
Fu, M. et al. Carbon dots: a unique fluorescent cocktail of polycyclic aromatic hydrocarbons. Nano Lett. 15, 6030–6035 (2015).
Righetto, M. et al. Spectroscopic insights into carbon dot systems. J. Phys. Chem. Lett. 8, 2236–2242 (2017).
Zhao, P. & Zhu, L. Dispersibility of carbon dots in aqueous and/or organic solvents. Chem. Commun. 54, 5401–5406 (2018).
Juzenas, P. et al. Quantum dots and nanoparticles for photodynamic and radiation therapies of cancer. Adv. Drug Deliv. Rev. 60, 1600–1614 (2008).
Hola, K. et al. Carbon dots—emerging light emitters for bioimaging, cancer therapy and optoelectronics. Nano Today 9, 590–603 (2014).
Luo, P. G. et al. Carbon-based quantum dots for fluorescence imaging of cells and tissues. RSC Adv. 4, 10791–10807 (2014).
LeCroy, G. E. et al. Functionalized carbon nanoparticles: syntheses and applications in optical bioimaging and energy conversion. Coord. Chem. Rev. 320–321, 66–81 (2016).
Yuan, F. et al. Shining carbon dots: synthesis and biomedical and optoelectronic applications. Nano Today 11, 565–586 (2016).
Sharma, V., Tiwari, P. & Mobin, S. M. Sustainable carbon-dots: recent advances in green carbon dots for sensing and bioimaging. J. Mater. Chem. B 5, 8904–8924 (2017).
Pardo, J., Peng, Z. & Leblanc, R. M. Cancer targeting and drug delivery using carbon-based quantum dots and nanotubes. Molecules 23, 378 (2018).
Du, J., Xu, N., Fan, J., Sun, W. & Peng, X. Carbon dots for in vivo bioimaging and theranostics. Small 15, 1805087 (2019).
Molaei, M. J. Carbon quantum dots and their biomedical and therapeutic applications: a review. RSC Adv. 9, 6460–6481 (2019).
Tian, Z. et al. Full-color inorganic carbon dot phosphors for white-light-emitting diodes. Adv. Opt. Mater. 5, 1700416 (2017).
Fernando, K. A. S. et al. Carbon quantum dots and applications in photocatalytic energy conversion. ACS Appl. Mater. Interfaces 7, 8363–8376 (2015).
Yu, H. et al. Smart utilization of carbon dots in semiconductor photocatalysis. Adv. Mater. 28, 9454–9477 (2016).
Wang, R., Lu, K. Q., Tang, Z. R. & Xu, Y. J. Recent progress in carbon quantum dots: synthesis, properties and applications in photocatalysis. J. Mater. Chem. A 5, 3717–3734 (2017).
Essner, J. B. & Baker, G. A. The emerging roles of carbon dots in solar photovoltaics: a critical review. Environ. Sci. Nano 4, 1216–1263 (2017).
Hu, C., Li, M., Qiu, J. & Sun, Y.-P. Design and fabrication of carbon dots for energy conversion and storage. Chem. Soc. Rev. 48,, 2315–2337 (2019).
Tang, L. et al. Deep ultraviolet photoluminescence of water-soluble self-passivated graphene quantum dots. ACS Nano 6, 5102–5110 (2012).
Tang, L. et al. Deep ultraviolet to near-infrared emission and photoresponse in layered N-doped graphene quantum dots. ACS Nano 8, 6312–6320 (2014).
Würth, C., Grabolle, M., Pauli, J., Spieles, M. & Resch-Genger, U. Relative and absolute determination of fluorescence quantum yields of transparent samples. Nat. Protoc. 8, 1535–50 (2013).
Kaiser, E., Colescott, R. L., Bossinger, C. D. & Cook, P. I. Color test for detection of free terminal amino groups in the solid-phase synthesis of peptides. Anal. Biochem. 34, 595–598 (1970).
Gaskell, K. & Ramsdell, D. AFM standard operating procedure. http://www.chem.umd.edu/wp-content/uploads/2013/05/AFM-SOP-ver2.pdf (2013).
Elgrishi, N. et al. A practical beginner’s guide to cyclic voltammetry. J. Chem. Educ. 95, 197–206 (2018).
Graham, D. J. Standard operating procedures for cyclic voltammetry. https://sop4cv.com/index.html.
Cardona, C. M., Li, W., Kaifer, A. E., Stockdale, D. & Bazan, G. C. Electrochemical considerations for determining absolute frontier orbital energy levels of conjugated polymers for solar cell applications. Adv. Mater. 23, 2367–2371 (2011).
Hwang, Y.-J., Courtright, B. A. E., Ferreira, A. S., Tolbert, S. H. & Jenekhe, S. A. 7.7% Efficient all-polymer solar cells. Adv. Mater. 27, 4578–4584 (2015).
Greenfield, N. J. Using circular dichroism spectra to estimate protein secondary structure. Nat. Protoc. 1, 2876–2890 (2007).
Garbett, N. C., Ragazzon, P. A. & Chaires, J. B. Circular dichroism to determine binding mode and affinity of ligand-DNA interactions. Nat. Protoc. 2, 3166–3172 (2007).
Tomassoli, I. & Gündisch, D. The twin drug approach for novel nicotinic acetylcholine receptor ligands. Bioorg. Med. Chem. 23, 4375–4389 (2015).
Moegling, J. et al. Bis(pyrazolyl)methane copper complexes as robust and efficient catalysts for Sonogashira couplings. Eur. J. Org. Chem. 2015, 7475–7483 (2015).
Cold Spring Harbor Laboratory. Formaldehyde (37%, w/v). Cold Spring Harb. Protoc. 2008, https://doi.org/10.1101/pdb.rec11372 (2008).
Acknowledgements
We thank all our colleagues, co-workers, and collaborators, whose names appear in the publications that constitute the basis of this protocol. This work was supported by the University of Trieste, INSTM, AXA Research Fund, the Spanish Ministry of Economy and Competitiveness MINECO (project CTQ2016-76721-R), Diputación Foral de Gipuzkoa program Red (101/16), ELKARTEK bmG2017 (ref: Elkartek KK-2017/00008, BOPV resolution: 8 February 2018) and the Maria de Maeztu Units of Excellence Program from the Spanish State Research Agency (MDM-2017-0720).
Author information
Authors and Affiliations
Contributions
L.Ð. and F.A. designed and performed the experiments and wrote the manuscript. M.P. planned the research, co-wrote the manuscript and secured the funding.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Protocols thanks Shaomin Shuang and other anonymous reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Key references using this protocol
Arcudi, F., Đorđević, L. & Prato, M. Angew. Chem. Int. Ed. 55, 2107–2112 (2016): https://doi.org/10.1002/anie.201510158
Carrara, S., Arcudi, F., Prato, M. & De Cola, L. Angew. Chem. Int. Ed. 56, 4757–4761 (2017): https://doi.org/10.1002/anie.201611879
Arcudi, F. Đorđević, L. & Prato, M. Angew. Chem. Int. Ed. 56, 4170–4173 (2017): https://doi.org/10.1002/anie.201612160
Arcudi, F. et al. Angew. Chem. Int. Ed. 56, 12097–12101 (2017): https://doi.org/10.1002/anie.201704544
Rizzo, C. et al. ACS Nano 12, 1296–1305 (2018): https://doi.org/10.1021/acsnano.7b07529
Rigodanza, F., Đorđević, L., Arcudi, F. & Prato, M. Angew. Chem. Int. Ed. 57, 5062–5067 (2018): https://doi.org/10.1002/anie.201801707
Ðorđević, L. et al. Nat. Commun. 9, 3442 (2018): https://doi.org/10.1038/s41467-018-05561-2
Integrated supplementary information
Supplementary Figure 1 Time-course illustration of key steps in the preparation of NCNDs.
(a) Reagents and reaction setup for the synthesis of NCNDs. (b) Weigh the arginine powder in a microwave vessel (Step 1). (c-e) Add stir bar and liquids (milli-Q H2O, followed by ethylenediamine) (Steps 2-4). (f-g) Cap the reaction vessel and mix the components (Steps 5-6). (h-j) Microwave-assisted synthesis of NCNDs (Step 7). (k) Reaction vessel after microwave irradiation (after Step 7). (l-p) Filter the crude reaction through a micro-filter (o shows the filtered solution under 365 nm light) (Steps 9-10A(i)). (r-u) Dialyze against pure water of the filtered solution (Step 10A(ii-v). (v-w) Powder NCNDs after freeze-drying the dialyzed solution (Step 10A(vii-viii)).
Supplementary Figure 2 Temperature, pressure and irradiation power monitored during the microwave-assisted synthesis of NCNDs.
(a) Temperature profile (°C). (b) Pressure profile psi). (d) Power profile (Watt).
Supplementary Figure 3 Temperature, pressure and irradiation power monitored during the microwave-assisted synthesis of QCNDs.
(a) Temperature profile (°C). (b) Pressure profile psi). (d) Power profile (Watt).
Supplementary Figure 4 Temperature, pressure and irradiation power monitored during the microwave-assisted synthesis of cNDI∙CNDs.
(a) Temperature profile (°C). (b) Pressure profile psi). (d) Power profile (Watt).
Supplementary Figure 5 Temperature, pressure and irradiation power monitored during the microwave-assisted synthesis of NCNDs-R.
(a) Temperature profile (°C). (b) Pressure profile psi). (d) Power profile (Watt).
Supplementary Figure 6 Characterization of ‘filter’ and ‘dialysate’.
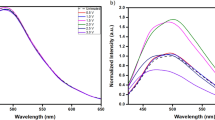
(a) Photographs of the filtration and dialysis steps, which samples were used for further characterization. (b) MALDI-TOF analysis of the NCNDs sample after dialysis for 48 h. (c) UV-Vis spectra in water (298 K). (d) Fluorescence spectra in water (298 K) of the sample left on the filter. (e) Fluorescence spectra in water (298 K) of the ‘dialysate’, which is the sample obtained by rotary evaporation concentration of the dialysate.
Supplementary information
Supplementary Information
Supplementary Figs. 1–6
Rights and permissions
About this article
Cite this article
Ðorđević, L., Arcudi, F. & Prato, M. Preparation, functionalization and characterization of engineered carbon nanodots. Nat Protoc 14, 2931–2953 (2019). https://doi.org/10.1038/s41596-019-0207-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41596-019-0207-x
This article is cited by
-
Fe3+ Sensing Based on Hydrogel Optical Fiber Doped with Nitrogen Carbon Dots
Journal of Electronic Materials (2024)
-
Synthesis, applications in therapeutics, and bioimaging of traditional Chinese medicine-derived carbon dots
Carbon Letters (2024)
-
Carbon Dots with Antioxidant Capacity for Detecting Glucose by Fluorescence and Repairing High-Glucose Damaged Glial Cells
Journal of Fluorescence (2024)
-
Dysprosium-doped carbon quantum dot nanocarrier: in vitro anticancer activity
Bulletin of Materials Science (2024)
-
Regulation of biological processes by intrinsically chiral engineered materials
Nature Reviews Materials (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.