Abstract
Zeolite fibers have attracted growing interest for a range of new applications because of their structural particularity while maintaining the intrinsic performances of the building blocks of zeolites. The fabrication of uniform zeolite fibers with tunable hierarchical porosity and further exploration of their catalytic potential are of great importance. Here, we present a versatile and facile method for the fabrication of hierarchical ZSM-5 zeolite fibers with macro-meso-microporosity by coaxial electrospinning. Due to the synergistic integration of the suitable acidity and the hierarchical porosity, high yield of propylene and excellent anti-coking stability were demonstrated on the as-prepared ZSM-5 hollow fibers in the catalytic cracking reaction of iso-butane. This work may also provide good model catalysts with uniform wall thickness and tunable porosity for studying a series of important catalytic reactions.
Similar content being viewed by others
Introduction
As an important class of crystalline aluminosilicates with uniform and ordered networks of micropores, zeolites have been widely used in the fields of catalysis, adsorption, separation and advanced materials1,2,3,4.The micropores endow zeolites with high specific surface area, excellent stability and unique shape selectivity. However, the molecular dimensions of micropores also make zeolites suffer from intracrystalline diffuse limitations5,6,7,8, leading to the slow diffusion and restricted mass transfer of the reactants and products, which affect the activity, selectivity and even lifetime of the zeolite catalysts in some important reactions9,10,11. Approaches for overcoming this problem include: preparing small-sized zeolite particles and ultra-thin zeolite nanosheets12,13,14,15, synthesizing new zeolites with larger pores16,17 and modifying the pore characteristics of zeolites18 etc. Among them, the synthesis of hierarchical zeolites with multiple levels of porosity and thus possessing combined advantages of the acidity and stability of micropores as well as the enhanced diffusion of larger pores, is considered one of the best approaches and has attracted increasing attention19,20,21,22,23,24,25,26,27,28,29,30.
The critical issue in the preparation of hierarchical zeolites is how to construct well-interconnected pores with multi-levels in a versatile and controlled manner and further tailor them for target applications. In this regard, great progress has been made in the past few decades. However, most of the work was focused on the bimodal porous structure on micro-/meso scale, which are still limited for efficient diffusion. Therefore, hierarchically macro-meso-microporous materials are expected to further enhance the mass transport and thus maximize the benefits of hierarchy in some catalyzed reactions31,32,33,34,35. As a type of unique morphology of material, zeolite fiber has attracted growing interest for a wide range of new applications in adsorption, optics and chemical sensors36,37,38, etc. Specifically in catalysis, compared with the conventional zeolite powders, the structured zeolite fibers could provide fast diffusion and low pressure drop while maintaining the intrinsic high performance of zeolite39,40,41. Although hierarchical zeolites and zeolite fibers have been studied, the reports on the fabrication of hierarchically macro-meso-microporous fibers with tunable macroporosity and further exploration of their catalytic application are rare.
Electrospinning is a simple and versatile method for the preparation of a wide variety of one-dimensional materials by using electrostatic force42,43,44,45. One notable progress of electrospinning technique is the coaxial electrospinning by the introduction of the coaxial nozzle, which could create core-shell or hollow fibers in one-step46,47,48,49,50. Herein, by assembling ZSM-5 nanocrystals via coaxial electrospinning, we successfully prepared high quality ZSM-5 zeolite hollow fibers with trimodal porosity in a large scale. The big advantages of the present assembing of zeolite nanocrystals, not only lie in that it provides a trimodal porosity and a good control of the wall thickness of the fibers, but also in that it presents a model catalyst system for studying the catalytic cracking of hydrocarbons. The presence of the acid sites and the hierarchical porosity renders them highly effective in enhancing the yield of propylene and the anti-coking stability during the cracking of iso-butane. To the best of our knowledge, this is the first report on the fabrication of the macro-meso-microporus ZSM-5 hollow fibers with tunable macroporosity and their application in catalytic cracking reaction. The present study is expected to provide new insights for the development of fibrous zeolite catalysts for applications in microreactors and structured zeolite beds.
Results and Discussion
Hierarchical ZSM-5 hollow fibers
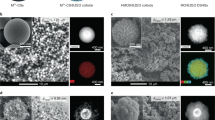
In the present coaxial electrospinning process, a suspension of ZSM-5 nanocrystals in polyvinylpyrrolidon (PVP)/ethanol solution worked as outer fluid and paraffin oil served as inner fluid. ZSM-5 nanocrystals were prepared by using tetrapropylammonium hydroxide (TPAOH) as the template according to the literature14. PVP used herein was to provide viscosity to make the outer fluid feasible for the fluent electrospinning and it also helped to bond the ZSM-5 nanocrystals together. Paraffin oil, which is immiscible with the PVP solution, was for presenting hollow channel via calcination. By optimizing the proportion of PVP (15 wt%) and ZSM-5(8 wt%), the ZSM-5/PVP composite fibers were successfully prepared (Figure 1a). The advantage of the present method lies in the capability and versatility for the preparation of the fibrous zeolites in a large scale. To obtain high quality and continuous non-woven mats of ZSM-5 zeolite fibers, the suitable calcination is also of great importance and temperature-programmed treatment was employed to remove the organic components of PVP, TPAOH and paraffin oil. Upon the removal of the organics via calcination at 550°C, high-quality ZSM-5 zeolite hollow fibers were obtained. Figure 1b shows the SEM image of the calcined hollow fibers. From Figure 1b, it can be clearly seen that the as-prepared fibers possess hollow structure with comparatively uniform diameter. The high magnification SEM image (Figure 1c) shows that the wall of the hollow fibers is rough and it consists of zeolite nanoparticles with the average particle size of 130 nm (see Figure S2). TEM image (Figure 1d) of the sample further confirms the continuous hollow channel character of the fibers. XRD pattern (Figure 1e) indicates that the product is typical of MFI topology. The nitrogen adsorption experiment was conducted to determine the textual properties of the zeolite fibers and the result is shown in Figure 1f. From Figure 1f, it can be seen that there is a high uptake at the initial stage of the isotherms, which is associated with the micropores in zeolites. The hysteresis loop at p/p0 > 0.2 in the sorption isotherms confirms the formation of mesopores. The mesopore size determined by BJH method was centered at 33 nm (Figure 1f, inset) which is attributed to the stacking pores of ZSM-5 nanocrystals of the wall of the hollow fibers (Figure S3). Therefore, macro-meso-microporous zeolite hollow fibers with micropores within the ZSM-5 nanoparticles, mesopores formed by the packing of the nanoparticles, uniform and continuous macropore on the hollow fiber level, are successfully demonstrated. And the hollow fibers possess good structural stability with no obvious destruction after ultrasonic treatment (Figure S4). In addition, by regulating feed rate of the inner fluid in coaxial electrospinning, the sizes of the macropore, i.e. the diameters of the hollow fibers can be facilely tuned. In the present experiments, it was found that changing the feed rate of the inner fluid from 0.4 to 1.2 mL·h−1 leads to the variation of the average inner diameter from about 1.59 μm to 2.26 μm (see Figure S5 in the supporting information).
(a) SEM image of the fibers that were co-electrospun from ZSM-5/PVP in ethanol solution with inner fluid of paraffin oil before calcination. (b) and (c) The low and magnified SEM image of the hollow fibers after calcination at 550°C, respectively. They show the high-quality hollow fibers. (d) TEM image of one hollow fiber after calicination. The wall thickness of the hollow fiber is relative uniform, which is composed of ZSM-5 nanoparticles. (e) XRD pattern of the ZSM-5 hollow fibers after calicination, indicative of the MFI topology. (f) Nitrogen adsorption/desorption isotherms and BJH pore size distribution of the ZSM-5 hollow fibers (inset), showing the meso-microporous characteristics.
Catalytic activity
To demonstrate the superiority in catalytic application, the catalytic performances of the hierarchical ZSM-5 hollow fibers were investigated in the cracking reaction of iso-butane to light olefins. The typical results were compared with those on hierarchical meso-microporous ZSM-5 fibers electrospun by the same ZSM-5 nanocrystals, ZSM-5 nanocrystals and conventional ZSM-5 zeolite (with the approximate Si/Al2 ratio of 100), respectively (see Figure S2, Figure S6, Figure S7, Table S1 in the supporting information). Table 1 presents the reactivities of conventional ZSM-5, Nano-ZSM-5, ZSM-5 solid fibers and ZSM-5 hollow fibers. From Table 1, it can be seen that in comparison with that on conventional ZSM-5 at 625°C, the conversion of the iso-butane was increased on hierarchical ZSM-5 samples including ZSM-5 fibers and Nano-ZSM-5. Especially at low temperatures (below 625°C), the hierarchical ZSM-5 zeolite fibers showed higher conversion of iso-butane and yield of propylene than those of other samples (Figure 2). Such results can be understood by analysis of the acid characterization result on NH3-TPD of the four samples (Figure 3) and the hierarchical pore of the zeolite fibers. From Figure 3, it can be seen that, compared with conventional ZSM-5 (Conv-ZSM-5), both the amount and the strengths of acid sites of hierarchical zeolites decreased and the percentage amount of the weak and medium acid in total amount of acid sites increased. It has been recognized that the construction of hierarchical pores is effective in enhancing the accessibility of the active centers51. In the two fibrous ZSM-5 samples, although the amount of the acid sites is not larger than that of conventional ZSM-5 zeolite, the presence of the mesopores and/or macropores make them have the enhanced diffusion and accessibility to catalytic active sites, which can partially compensate for the low acidity strength of the two samples. Consequently, although the acid strength is not improved, the conversion of iso-butane is increased. The increase of percentage of the amount of the medium and weak acid sites and the decrease of the acid strength result in the enhancement of selectivity to propylene, which is mainly related to the fact that propylene is the intermediate product in the cracking of iso-butane and the suitable strength and amount of acid sites is one of the key factors for making the reaction proceed the exact extent and for hindering propylene from secondary reactions52. Among the four ZSM-5 samples, ZSM-5 fiber shows the highest yield of propylene by 42.0%, which is 4.3% higher than that on conventional ZSM-5.
Figure 4 presents the stability test results of the four catalysts by monitoring the maximum yield of propylene during 60 h continuous reactions at 625°C. From Figure 4a, it can be seen that during 60 h on stream, ZSM-5 hollow fibers exhibited the highest stability among the four tested samples for the cracking of iso-butane. Even after 60 hours of continuous reaction, the conversion of iso-butane on ZSM-5 hollow fiber sample still retained at 93.3%, which was 12.1% and 5.5% higher than those on ZSM-5 solid fibers and Nano-ZSM-5, respectively. For ZSM-5 hollow fibers, it is noteworthy that even though the conversion decreased slightly with the reaction time, the yield of propylene was still about 40% (Figure 4b), indicating that the selectivity to propylene was increased. It is known that the long time reaction brings about the coking of the catalyst and the coking occurs more serious on the stronger acid sites52,53,54. Considering this and comparing the reaction stability between Nano-ZSM-5 and Conv-ZSM-5, because Conv-ZSM-5 possesses higher strength and larger amount of strong acid sites as well as the larger particle size, it exhibits worse anti-coking than Nano-ZSM-5. While ZSM-5 hollow fibers show the best anti-coking which possesses the comparable acidic properties with those of nano-ZSM-5 and ZSM-5 solid fibers, indicating that its unique porosity does play the key role, i.e. the presence of interconnected hierarchical macro-meso-micropores facilitates the mass transfer of target products and coke precursor out of the micropores. Coking carbon distribution of the conventional ZSM-5 particle and ZSM-5 hollow fiber after reactions via EDX line scans (Figure S8) supports this suggestion. The typical result shows that along the cross-section of the conventional ZSM-5 particle, a decreased carbon deposition occurred from the outer to the interior part. While a homogeneous carbon coking appears on ZSM-5 hollow fiber, indicating that compared with conventional ZSM-5 catalyst, the ZSM-5 hollow fiber does not suffer from the diffusion limitation during the present reaction. So, it can be concluded that the synergistic effect of the suitable acidity and hierarchical pores contribute to the fact that the catalytic performances of macro-meso-microporous ZSM-5 hollow fibers are the best among the four kind of samples. In addition to the good performances of the ZSM-5 hollow fibers, such facile regulation of the hierarchical porosity with uniform wall thickness by assembling of ZSM-5 nanocrystals in the present study also helps to provide good model catalysts for the deep studying of the catalytic cracking of hydrocarbons.
Catalytic stability of the test results of conventional ZSM-5 (Conv-ZSM-5), Nano-ZSM-5, ZSM-5 solid fibers and ZSM-5 hollow fibers during 60 h on stream at 625°C by monitoring the conversion of iso-butane (a) and the yield of propylene (b).
ZSM-5 hollow fibers show the best anti-coking performance among the four samples.
In summary, we have presented a facile method for the fabrication of hierarchical ZSM-5 fibers with macro-meso-microporosity. Uniform and tunable macropores were realized for the first time on such a kind of fibrous zeolite by regulating the rates of inner fluid in coaxial electrospinning. The as-prepared hierarchical ZSM-5 hollow fibers exhibit high yield of propylene and good anti-coking stability in the cracking reaction of iso-butane. The excellent catalytic performances are attributed to the combined effect of the suitable acidity and the hierarchical porosity on the promotion of the diffusion and the accessibility to the catalytic active sites. The current work is expected to provide new insight on the design and expanding the application of hierarchical fibrous zeolite systems in a range of important catalytic reactions.
Methods
Synthesis of ZSM-5 nanocrystals
ZSM-5 nanocrystals were prepared from a clear solution with the composition of 9TPAOH: 0.25Al2O3: 25SiO2: 599H2O according to previous literature14 except that the reaction was conducted at 100°C for 84 h. The synthesized nanocrystals were purified by centrifugation and washed with distilled water until the pH value close to 7.0. Finally, the resultant powder were washed twice with ethanol and dried at 80°C overnight for further use in oven.
Preparation of outer fluid
In order to form a suspension with an appropriate concentration, the synthesized ZSM-5 nanocrystals were subjected to ultrasonic treatment (KS Ultrasonic Instruments KQ-200VDE, Max Power 200 W) at 180 W for 6 h to re-disperse into absolute ethanol. Then measured amount of polyvinylpyrrolidone (PVP, Aldrich, Mw ≈ 1,300,000) was added under stirring to get an appropriate viscosity. When the PVP was completely dissolved, the opalescent solution obtained was processed by ultrasonic again to exclude blended bubble in the process of mixing. After the treatment, the electrospinning solution was obtained with uniformity and moderate viscosity.
In a typical experiment, the preparation of outer fluid was achieved by the addition of 2.50 g (8 wt%) dry ZSM-5 nanocrystals into 24.06 g absolute ethanol in a sealable bottle and the mixture was sonicated for 6 h. Then, under magnetically stirring, 4.70 g PVP (15 wt%) powder was added into the suspension solution and it was further stirred overnight to completely dissolve the PVP molecules. Finally, the obtained solution was sonicated at 180 W for another 0.5 h.
Preparation of hierarchical ZSM-5 fibers
Schematic illustration of the coaxial electrospinning device is shown in Figure S1, which is similar to that described in the literature50. For the fabrication of ZSM-5 hollow fibers, the paraffin oil as inner liquid was pumped out from the inner metallic nozzle connected by 20.0 mL volume syringe with flow rate of 0.4 ~ 2.0 mL h−1 and the prepared solution (5.0 mL) as outer liquid was pumped through annular space of concentric metallic needle with the flow rate of 4.5 mL h−1. The process of electrospinning was carried out by applying an 18 ~ 25 kV voltage at a distance of 30 cm to aluminum foil as collector to receive non-woven mats of ZSM-5/PVP composite fibers. The electrospun products were calcined under air at a heating rate of 1°C min−1 up to 550°C and kept at the temperature for 6 h to burn off the organic polymer, paraffin oil and the structure-directing agent (tetrapropylammonium hydroxide). As control, the solid fibers were fabricated by single nozzle electrospinning without inner fluid and the conventional ZSM-5 zeolite (Si/Al2 ratio of 100), was purchased from Catalyst Company of NanKai University.
References
Davis, M. E. Ordered porous materials for emerging applications. Nature 417, 813–821 (2002).
Cundy, C. S. & Cox, P. A. The hydrothermal synthesis of zeolites: history and development from the earliest days to the present Time. Chem. Rev. 103, 663–702 (2003).
Lew, C. M., Cai, R. & Yan, Y. S. Zeolite thin films: from computer chips to space stations. Acc. Chem. Res. 43, 210–219 (2010).
Meng, X. J. & Xiao, F. S. Green routes for synthesis of zeolites. Chem. Rev. 114, 1521–1543 (2014).
Corma, A. From microporous to mesoporous molecular sieve materials and their use in Catalysis. Chem. Rev. 97, 2373–2420 (1997).
van Donk, S., Janssen, A. H., Bitter, J. H. & de Jong, K. P. Generation, characterization and impact of mesopores in zeolite catalysts. Catal. Rev.: Sci. Eng. 45, 297–319 (2003).
Pérez-Ramírez, J., Christensen, C. H., Egeblad, K., Christensen, C. H. & Groen, J. C. Hierarchical zeolites: enhanced utilisation of microporous crystals in catalysis by advances in materials design. Chem. Soc. Rev. 37, 2530–2542 (2008).
Bellussi, G., Carati, A., Rizzo, C. & Millini, R. New trends in the synthesis of crystalline microporous materials. Catal. Sci. Technol. 3,833–857 (2013).
Zhao, L., Shen, B. J., Gao, J. S. & Xu, C. M. Investigation on the mechanism of diffusion in mesopore structured ZSM-5 &improved heavy oil conversion. J. Catal. 258, 228–234 (2008).
Li, X. F., Prins, R. & Van Bokhoven, J. A. Synthesis and characterization of mesoporous mordenite. J. Catal. 262, 257–265 (2009).
Christensen, C. H., Johannsen, K., Schmidt, I. & Christensen, C. H. Catalytic benzene alkylation over mesoporous zeolite single crystals: improving activity and selectivity with a new family of porous materials. J. Am. Chem. Soc. 125, 13370–13371 (2003).
Choi, M. et al. Stable single-unit-cell nanosheets of zeolite MFI as active and long-lived catalysts. Nature 461, 246–249 (2009).
Na, K. et al. Directing zeolite structures into hierarchically nanoporous architectures. Science 333, 328–332 (2011).
Mintova, S. et al. Closely packed zeolite nanocrystals obtained via transformation of porous amorphous silica. Chem. Mater. 16, 5452–5459 (2004).
Jia, C. J., Liu, Y., Schmidt, W., Lu, A. H. & Schüth, F. Small-sized HZSM-5 zeolite as highly active catalyst for gas phase dehydration of glycerol to acrolein. J. Catal. 269, 71–79 (2010).
Corma, A., Díaz-Cabañas, M. J., Martínez-Triguero, J., Rey, F. & Rius, J. A large-cavity zeolite with wide pore windows and potential as an oil refining catalyst. Natue 418, 514–517 (2002).
Bu, X. H., Feng, P. Y. & Stucky, G. D. Large-cage zeolite structures with multidimensional 12-ring channels. Science 278, 2080–2085 (1997).
Wakui, K. et al. Catalytic cracking of n-butane over rare earth- loaded HZSM-5 catalysts. Stud. Surf. Sci. Catal. 125, 449–456 (1999).
Zhu, J., Cui, Y., Wang, Y. & Wei, F. Direct synthesis of hierarchical zeolite from a natural layered material. Chem. Comm. 45, 3282–3284 (2009).
Li, W. C. et al. Hierarchically structured monolithic silicalite-1 consisting of crystallized nanoparticles and its performance in the beckmann rearrangement of cyclohexanone oxime. J. Am. Chem. Soc. 127, 12595–12600 (2005).
Ogura, M. et al. Formation of uniform mesopores in ZSM-5 zeolite through treatment in alkaline solution. Chem. Lett. 29, 882–883 (2000).
Tao, Y. S., Kanoh, H., Abrams, L. & Kaneko, K. Mesopore-modified zeolites: preparation, characterization and applications. Chem. Rev. 106, 896–910 (2006).
Groen, J. C. et al. Direct demonstration of enhanced diffusion in mesoporous ZSM-5 zeolite obtained via controlled desilication.J. Am. Chem. Soc. 129, 355–360 (2007).
Jacobsen, C. J. H., Madsen, C., Houzvicka, J., Schmidt, I. & Carlsson, A. Mesoporous zeolite single crystals. J. Am. Chem. Soc. 122, 7116–7117 (2000).
Fan, W. et al. Hierarchical nanofabrication of microporous crystals with ordered mesoporosity. Nat. Mater. 7, 984–991(2008).
Yang, Z. X., Xia, Y. D. & Mokaya, R. Zeolite ZSM-5 with unique supermicropores synthesized using mesoporous carbon as a template. Adv. Mater. 16, 727–732 (2004).
Tosheva, L., Valtchev, V. & Sterte, J. Silicalite-1 containing microspheres prepared using shape-directing macro-templates. Micropor.Mesopor.Mater. 35–36, 621–629 (2000).
Xiao, F. S. et al. Catalytic properties of hierarchical mesoporous zeolites templated with a mixture of small organic ammonium salts and mesoscale cationic polymers. Angew. Chem. Int. Ed. 45, 3090–3093 (2006).
Wang, H. & Pinnavaia, T. J. MFI zeolite with small and uniform intracrystal mesopores. Angew. Chem. Int. Ed. 45, 7603–7606 (2006).
Chen, L. H. et al. Hierarchically structured zeolites: synthesis, mass transport properties and applications. J. Mater. Chem. 22, 17381–17403 (2012).
Ocampo, F., Yun, H. S., Pereira, M. M., Tessonnier, J. P. & Louis, B. Design of MFI zeolite-based composites with hierarchical pore structure: a new generation of structured catalysts. Cryst. Growth Des. 9, 3721–3729 (2009).
Tan, Q. F. et al. Synthesis, characterization and catalytic properties of hydrothermally stable macro-meso-micro-porous composite materials synthesized via in situ assembly of preformed zeolite Y nanoclusters on kaolin. J. Catal. 251, 69–79 (2007).
Xu, L. et al. Synthesis, characterization of hierarchical ZSM-5 zeolite catalyst and its catalytic performance for phenol tert-butylation reaction. Catal. Comm. 9, 1272–1276 (2008).
Chen, L. H. et al. Highly stable and reusable multimodal zeolite TS-1 based catalysts with hierarchically interconnected three-level micro-meso-macroporous structure. Angew. Chem. Int. Ed. 50, 11156–11161 (2011).
Yang, X. Y. et al. Well-organized zeolite nanocrystal aggregates with interconnected hierarchically micro-meso-macropore system showing enhacnced catalytic performance. Chem. Eur. J. 17, 14987–14995 (2011).
Goergen, S., Saada, M. A., Soulard, M., Rouleau, L. & Patarin, J. Dry gel conversion synthesis of shape controlled MFI type zeolite materials. J. Porous Mater. 17, 635–641 (2010).
Zhang, J., Luo, M., Xiao, H. & Dong, J. H., Interferometric study on the adsorption-dependent refractive index of silicalite thin films grown on optical fibers. Chem. Mater. 18, 4–6 (2006).
Wang, Z. B., Ge, Q. Q., Shao, J. & Yan, Y. S. High performance zeolite LTA pervaporation membranes on ceramic hollow fibers by dip coating-wiping seed deposition. J. Am. Chem.Soc. 131, 6910–6911 (2009).
Shen, K., Qian, W. Z., Wang, N., Su, C. & Wei, F. Fabrication of c-Axis oriented ZSM-5 hollow fibers based on an in situ solid−solid transformation mechanism. J. Am. Chem. Soc. 135, 15322–15325 (2013).
Di, J. C., Zhao, Y. & Yu, J. H. Fabrication of molecular sieve fibers by electrospinning. J. Mater. Chem. 21, 8511–8520 (2011).
Liu, B. S., Tang, D. C. & Au, C. T. Fabrication of analcime zeolite fibers by hydrothermal synthesis. Micropor. Mesopor. Mater. 86, 106–111 (2005).
Li, D. & Xia, Y. N. Electrospinning of nanofibers: reinventing the wheel. Adv. Mater. 16, 1151–1170 (2004).
Jiang, L., Zhao, Y. & Zhai, J. A Lotus-leaf-like superhydrophobic surface: a porous microsphere/nanofiber composite film prepared by electrohydrodynamics. Angew. Chem. Int. Ed. 43, 4338–4341 (2004).
Greiner, A. & Wendorff, J. H. Electrospinning: a fascinating method for the preparation of ultrathin fibers. Angew. Chem. Int. Ed. 46, 5670–5703 (2007).
Lu, X., Wang, C. & Wei, Y. One-dimensional composite nanomaterials: synthesis by electrospinning and their applications. Small. 5, 2349–2370 (2009).
Loscertales, I. G. et al. Micro/nano encapsulation via electrified coaxial liquid jets. Science 295, 1695–1698 (2002).
McCann, J. T., Marquez, M. & Xia, Y. Melt coaxial electrospinning: a versatile method for the encapsulation of solid materials and fabrication of phase change nanofibers. Nano. Lett. 6, 2868–2872 (2006).
Yu, J. H., Fridrikh, S. V. & Rutledge, G. C. Production of submicrometer diameter fibers by two-fluid electrospinning. Adv. Mater. 16, 1562–1566 (2004).
Zhao, Y., Cao, X. Y. & Jiang, L. Bio-mimic multichannel microtubes by a facile method. J. Am. Chem. Soc. 129, 764–765 (2007).
Di, J. C. et al. Fabrication of zeolite hollow fibers by coaxialelectrospinning. Chem. Mater. 20, 3543–3545 (2008).
Qin, Z. et al. Chemical equilibrium controlled etching of MFI-Type zeolite and its influence on zeolite structure, acidity and catalytic activity. Chem. Mater. 25, 2759–2766 (2013).
Jiang, G. Y. et al. Highly effective P-modified HZSM-5 catalyst for the cracking of C4 alkanes to produce light olefins. Appl. Catal. A: Gen. 340, 176–182 (2008).
Zhao, G. L. et al. Effect of phosphorus on HZSM-5 catalyst for C4-olefin cracking reactions to produce propylene. J. Catal. 248, 29–37 (2007).
Zhu, X. X. et al. Two new on-purpose processes enhancing propene production: catalytic cracking of C4 alkenes to propene and metathesis of ethene and 2-butene to propene. Catal. Surv. Asia. 13, 1–8 (2009).
Acknowledgements
The authors thank the support of this work by the National Basic Research Program of China (973 Program, No. 2012CB215001), National Science Foundation of China (Grant No. U1162117, 21222309), Beijing Nova Program (Grant No. Z11111005450000), Program for New Century Excellent Talents in University (NCET-11-0732), PetroChina Innovation Foundation (2011D-5006-0403) and the Fundamental Research Funds for the Central Universities.
Author information
Authors and Affiliations
Contributions
Jia Liu, Y.L., J.D. and Y.W. carried out the experiments and collected the data. G.J., Z.Z. and Y.Z. designed the project, analyzed the data and wrote the paper. Q.S., C.X., J.G., A.D., Jian Liu, Y.W. and L.J. discussed the results and commented on the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Supplementary Information
Supporting information
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License. The images or other third party material in this article are included in the article's Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder in order to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
About this article
Cite this article
Liu, J., Jiang, G., Liu, Y. et al. Hierarchical Macro-meso-microporous ZSM-5 Zeolite Hollow Fibers With Highly Efficient Catalytic Cracking Capability. Sci Rep 4, 7276 (2014). https://doi.org/10.1038/srep07276
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep07276
This article is cited by
-
Ce-doped TiO2-zeolite fibers: visible light-driven photocatalysts for indigo dye degradation
Environmental Science and Pollution Research (2023)
-
Improved aromatic yield and toluene selectivity in propane aromatization over Zn–Co/ZSM-5: effect of metal composition and process conditions
Journal of Porous Materials (2023)
-
Progress of Fabrication and Applications of Electrospun Hierarchically Porous Nanofibers
Advanced Fiber Materials (2022)
-
Zinc Modified Hierarchical ZSM-5 for Aromatisation of Propane
Journal of Porous Materials (2022)
-
Highly selective and stable Zn–Fe/ZSM-5 catalyst for aromatization of propane
Applied Petrochemical Research (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.