Abstract
The KMg4(PO4)3:Eu2+ phosphor was prepared by the conventional high temperature solid-state reaction. The crystal structure, luminescence and reflectance spectra, thermal stability, quantum efficiency and the application for N-UV LED were studied respectively. The phase formation and crystal structure of KMg4(PO4)3:Eu2+ were confirmed from the powder X-ray diffraction and the Rietveld refinement. The concentration quenching of Eu2+ in the KMg4(PO4)3 host was determined to be 1mol% and the quenching mechanism was certified to be the dipole–dipole interaction. The energy transfer critical distance of as-prepared phosphor was calculated to be about 35.84Å. Furthermore, the phosphor exhibited good thermal stability and the corresponding activation energy ΔE was reckoned to be 0.24eV. Upon excitation at 365nm, the internal quantum efficiency of the optimized KMg4(PO4)3:Eu2+ was estimated to be 50.44%. The white N-UV LEDs was fabricated via KMg4(PO4)3:Eu2+, green-emitting (Ba,Sr)2SiO4:Eu2+ and red-emitting CaAlSiN3:Eu2+ phosphors with a near-UV chip. The excellent color rendering index (Ra = 96) at a correlated color temperature (5227.08K) with CIE coordinates of x = 0.34, y = 0.35 of the WLED device indicates that KMg4(PO4)3:Eu2+ is a promising blue-emitting phosphor for white N-UV light emitting diodes (LEDs).
Similar content being viewed by others
Introduction
Since the first light emitting diode (LED) light source was invented by Nick Holonyak of General Electric, it has drawn more and more attention to apply in solid-state lighting and create an enormous revolution on the lighting industry. Recently, a great attention has been focused on white LEDs as solid-state lighting and as components of display devices because of their low energy consumption, high efficiency, long operational lifetime (>100 000h), environmental friendliness and high material stability1,2,3,4. There are several ways to assemble the white LEDs. The most prevalent strategy is produced by pumping the blue InGaN chip with yellow-emitting Y3Al5O12:Ce3+ (YAG) phosphor. Nevertheless, high correlated color temperature (CCT) and low color rendering (CRI) index (Ra < 80) restrict it to provide sunlight-like illumination due to the deficiency in red emission. In order to generate excellent CRI values and appropriate CCT white light for display or general illumination light sources, the method of pumping blue, green and red-emitting phosphors with near ultraviolet (N-UV) LEDs has been investigated5,6,7. Thereinto, the BaMgAl10O17:Eu2+ (BAM), which is the most commonly used commercial blue phosphor for N-UV LEDs as high efficiency, suffer from poor thermal stability4,8,9. Accordingly, the development of excellent structure and thermal stability of blue phosphor host for N-UV LEDs is highly desirable.
Eu2+ ion is the most frequently used blue-emitting activator in phosphor, which shows broad N-UV excitation and visible emission in a specific host owing to the 4f–5d transitions. According to the impact of the strength of the crystal field and covalent, Eu2+ can emit light from the ultraviolet to the infrared with broadband emitting fluorescence in different matrixes and the corresponding fluorescence lifetimes locate commonly in the range of 0.2–2.0μs10,11. On basis of these characteristics, multifarious Eu2+ -activated phosphors have been widely studied in LED lighting, 3D displays and scintillators in detection devices.
The phosphate KMg4(PO4)3 compound was first obtained from flux during crystallizing K2MgWO2(PO4)2 by Tomaszewski and co-workers12. Xiaofeng Lan et al. reported the luminescence properties of Eu2+-activated KMg4(PO4)3 by combustion-assisted synthesis method in 201213. However, to our best knowledge, the temperature-dependent luminescence characteristics as well as the application of KMg4(PO4)3:Eu2+ pumped for n-UV LEDs have not been investigated. In this paper, the KMg4(PO4)3:Eu2+ phosphor was firstly prepared by the conventional high temperature solid-state reaction method. The crystal structure, reflectance spectra, thermal stability, quantum efficiency and applications in white NUV LED are studied respectively. White LEDs was fabricated by combing an N-UV LED chip (λmax = 385nm) with the KMg4(PO4)3:Eu2+, along with green and red phosphors and its optical properties have also been investigated. The results demonstrate that the blue-emitting KMg4(PO4)3:Eu2+ is a promising blue-emitting phosphor for white N-UV LEDs.
Results
XRD Refinement and Crystal Structure
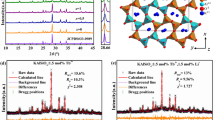
Figure 1 depicts the XRD patterns of the series of as-synthesized KMg4(PO4)3:xEu2+ (x = 0, 0.02, 0.06 and 0.1) and the standard pattern (JCPDS 15-4111) of KMg4(PO4)3 is shown as a reference. It can be found from the Figure 1 that all XRD patterns agree well with the standard pattern and no other phase is observed, which demonstrates that the single phase of KMg4(PO4)3:xEu2+ was obtained and the doping of Eu2+ ions did not cause any notable impurities or any structural variation. Besides, the main diffraction peaks shift slightly to the higher angle side with increasing Eu2+ concentrate, as shown in Figure 1(b). This observation means that the lattice was distorted by substitution the ions which are comparatively big radius in KMg4(PO4)3 host lattice with Eu2+ ions14. Thus, it is reasonable to assume that Eu2+ (r = 1.25Å for coordinate number (CN) = 8 and r = 1.17Å for CN = 6) ions substituted the position of K+ sites (r = 1.51Å for CN = 8) because both the Mg2+ (r = 0.72Å for CN = 6 and r = 0.66Å when CN = 5) and P5+ (r = 0.17Å for CN = 4) sites are smaller than the Eu2+ ions15,16. For further understanding the phase purity and the occupancy of Eu2+ ions on K+ sites in KMg4(PO4)3:Eu2+, the Rietveld refinement of KMg4(PO4)3 and KMg4(PO4)3:0.06Eu2+ phosphors were analyzed via the GSAS program as shown in Figure 2. The KMg4(PO4)3 was served as an initial structural model. The results of Rietveld refinement further demonstrate that neither the host nor the doped 0.06mol Eu2+ ions generated any impurity or secondary phases in KMg4(PO4)3. The KMg4(PO4)3:xEu2+ belongs to an orthorhombic structure with the space group Pnnm(58). For the crystal of KMg4(PO4)3 host, the lattice parameters were fitted to be a = 16.3707(7)Å, b = 9.5627(4)Å, c = 6.1667(5)Å, cell volume (V) = 965.361(23)Å3 and the weighted profile R-factor (Rwp), the expected R factor (Rp) are 8.86% and 6.86%, respectively. As doped with Eu2+, the lattice parameters of KMg4(PO4)3:0.06Eu2+ became a = 16.3563(4)Å, b = 9.5570(2)Å, c = 6.1663(1)Å and V = 963.904(67)Å3. The refinement data converged to Rwp = 10.54% and Rp = 7.97%, as summarized in Table 1. The volumetric constriction with increasing Eu2+ doping concentration also indicates that the Eu2+ occupied the K+ ions sites.
Figure 3 illustrates the crystal structure of KMg4(PO4)3 and the coordination environment of the K+ ions. The compound of KMg4(PO4)3 is consist of PO4 tetrahedra, MgO6 octahedra and MgO5 polyhedra which are linked by P–O–Mg bridges. The K+ ions are located in the tunnels along b-axis and surrounded by the three-dimensional framework forming via interconnected polyhedra. As presented in Figure 3c, the K+ ions in KMg4(PO4)3 are eight-fold coordinated by oxygen ions with 4g position and m site symmetry.
Reflectance and Photoluminescence properties of the KMg4(PO4)3:xEu phosphor at RT
The reflectance spectra of KMg4(PO4)3 host and KMg4(PO4)3:0.05Eu2+ are presented in Figure 4a. The KMg4(PO4)3 host shows an energy absorption band ranging from 200 to 300nm and a high reflection ranging from 300 to 700nm. The band gap of the virgin KMg4(PO4)3 is calculated by using the following formula:17

in which hν means the energy per photon, C is a proportional constant and Eg represents the value of the band gap, n = 1/2 stands for an indirect allowed transition, 2 means a direct allowed transition, 3/2 represents a direct forbidden transition, or 3 indicates an indirect forbidden transion, R∞ = Rsample/Rstandard. The F(R∞) means the Kubelka−Munk function which can be formulated to the following equation:

where K, S and R represent the absorption, scattering and reflectance parameter, respectively. As illustrated in Figure 4b, the band gap energy of KMg4(PO4)3 host is estimated to be about 5.74eV from the extrapolation of the line for [F(R∞)hν]2 = 0. As Eu2+ ions were introduced into the host, strong broad absorption appeared in the 250–400nm N-UV range, which is matched well with the excitation spectrum.
(a) Excitation and emission spectra of KMg4(PO4)3:0.06Eu2+ (λem = 450nm for excitation and λex = 300 and 365nm for emission); Diffuse reflection spectra of KMg4(PO4):xEu2+ (x = 0 and 0.06); (b) Absorption spectra of KMg4(PO4)3:0.06Eu2+ matrix calculated by the Kubelka−Munk equation. All spectra were taken at RT.
The photoluminescence emission (PL, λex = 300 and 365nm) and excitation (PLE, λem = 450nm) spectra of KMg4(PO4)3:0.06Eu2+ are also depicted in Figure 4a. The PLE spectrum of KMg4(PO4)3:0.06Eu2+ presents a broad hump ranging at 250–400nm, which originates from the 4f7–4f65d transition of Eu2+ ions. It indicates that the broad excitation spectrum of KMg4(PO4)3:Eu2+ matches well with the emission of the commercial N-UV chip (365–420nm). The PL spectra of the KMg4(PO4)3:0.06Eu2+ phosphor present a 52.2nm full width at half-maximum (FWHM) broad blue emission band extending from 400 to 525nm peaking at 450nm, which is assigned to the 4f65d–4f7 transition of the Eu2+ ions. Moreover, The PL of KMg4(PO4)3:0.06Eu2+ detected under 365nm is similar to that under 300nm in addition to the difference of the relative intensity, which verifies that the Eu2+ ions occupy the same lattice site (K+ sites) in KMg4(PO4)3 host18. Above result is in accord with the conclusion from Rietveld refinement. The CIE chromaticity coordinates of KMg4(PO4)3:0.01Eu2+ and commercial BAM phosphors under 365nm UV excitation are illustrated in Figure 5. The color coordinates of KMg4(PO4)3:0.01Eu2+ and BAM are calculated to be (x = 0.1507, y = 0.0645) and (x = 0.1471, y = 0.0628), respectively. The inset shows the digital photograph of KMg4(PO4)3:0.01Eu2+ phosphor under a 365nm UV lamp, which indicates KMg4(PO4)3:Eu2+ phosphor can be used as a blue-emitting phosphor for w-LEDs application.
As Figure 4a shows, the PL and PLE spectra of KMg4(PO4)3:0.06Eu2+ overlap partially, which demonstrates the existence of energy transfer between Eu2+-Eu2+ 19. As we know, two main mechanisms can be explanatory for the resonant energy-transfer: exchange interaction or electric multipolar interaction20. In order to further investigate the process of energy transfer between activators or between sensitizer and activator, the Eu2+ concentration-dependent PL spectra of KMg4(PO4)3:xEu2+ (x = 0.005, 0.01, 0.02, 0.04, 0.06, 0.08) phosphors under 365nm light excitation are shown in Figure 6. The optimal doping concentration of Eu2+ for the PL intensity in KMg4(PO4)3:xEu2+ is 1% mol. When the doping content of Eu2+ exceeded 0.01mol%, the PL intensity began to decrease because of the concentration quenching effect which is due to the energy consumed via energy transfer from one activator to another21. Thus, the critical distance (Rc) for energy transfer among Eu2+ is necessary to obtain for further understanding the concentration quenching interaction mechanism. The value of the critical distance (Rc) can be reckoned via the following equation:22

where V means the unit cell volume, xc represents the concentration of activator ion where the quenching occurs and N is the number of the K+ ion in per unit cell. For the KMg4(PO4)3 host, xc = 0.01, N = 4 and V = 965.361Å3, hence, the value of Rc is calculated to about 35.84Å. Owing to the typical critical distance of the exchange interaction is about 5Å and the exchange interaction only fits the energy transfer of forbidden transitions5,23. Therefore, the electric multipolar interactions are dominant in the energy transfer process. The interaction type can be estimated via the following equation:24

in which x is the concentration of activation, which is not less than the critical concentration, I/x is the emission intensity (I) per activator concentration (x); K and β are constants under the same excitation condition of host lattice; and θ is a function of electric multipolar character. θ = 6, 8, 10 for dipole–dipole (d–d), dipole–quadrupole (d–q), quadrupole–quadrupole (q–q) interactions, respectively. In order to estimate the θ value, the dependence of lg(I/x) on lg(x) is illustrated in the inset of Figure 6. A relatively linear relation can be observed and the slope of the straight line is fitting to −1.4911 which equals to −θ/3. Hence, the value of θ is 4.4733, which is close to 6, demonstrating that the interaction type in KMg4(PO4)3:Eu2+ is dipole-dipole interactions.
To further explore the energy transfer process, the room temperature luminescence decay curves of Eu2+ ions in KMg4(PO4)3:xEu2+ (x = 0.005, 0.01, 0.02, 0.04, 0.06, 0.08; λex = 365nm, λem = 450nm) were measured, as shown in Figure 7. The decay curves can be fitted with an approximate single-exponential decay model as:

where I0 represents the initial emission intensity when t is 0 and τ means the lifetime. The average lifetimes of Eu2+ ions of KMg4(PO4)3:xEu2+ (x = 0.005, 0.01, 0.02, 0.04, 0.06, 0.08) were estimated to be 1.246, 1.262, 1.245, 1.176, 1.163 and 1.161μs, respectively. All measured decay times are reasonable for the 5d–4f allowed transition of Eu2+ in solids (~1μs). Besides, the decay time increases to maximum, when the concentration of Eu2+ increases to 0.01 and then the decay time reduces acutely, indicating an efficient energy transfer between Eu2+ ions and causing concentration quenching25,26.
Influence of Temperature on emission intensity and FWHM
The thermal quenching of luminescence is one of important technological parameters to be considered for phosphor materials applied in high power LEDs, because it significantly affects the light output and service life. The temperature-dependent PL spectra of the KMg4(PO4)3:0.01Eu2+ exited by 365nm N-UV light is depicted in Figure 8. With increasing temperature (30°C–300°C), the emission intensity decreases from 100% to 74.26% of that at 30°C and the FWHM of the emission band increases from 50.34 to 60.42nm. The inset of Figure 8 illustrates the comparison of the thermal luminescence quenching of KMg4(PO4)3:0.01Eu2+ with that of commercial BAM:Eu2+ and the FWHM of KMg4(PO4)3:0.01Eu2+ emission as a function of the temperature. As shown in Figure 8, it can be found only 9% decay at 150°C for KMg4(PO4)3:0.01Eu2+, which indicates that the thermal stability of KMg4(PO4)3:Eu2+ is superior to that of commercial BAM:Eu2+ below 200°C and this phosphor could be used as a promising phosphor for high-power LED application. To better understand the thermal quenching process, the configurational coordinate diagram can be used to respond to this phenomenon. As the temperature increases, the interaction of electron-phonon is intensive. Along with the enhancing of phonon interaction, more electrons can be thermally activated to the crossover between the 4f65d excited state and 4f7 ground state, whereupon release the energy by generating lattice vibration22. The excellent thermal stability of KMg4(PO4)3:Eu2+ may relate to the indurative structure which is combined with PO4 tetrahedra, MgO6 octahedra and MgO5 polyhedra via P-O-Mg bridges to form the three-dimensional framework. In this indurative structure, the energy of the electrons in the excited state is difficult to release via lattice vibration, which means smaller Stokes shift in a configurational coordinate diagram model and higher activation energy (ΔE)27. The activation energy can be estimated by using the Arrhenius equation:28

in which I0 and I are the luminescence intensity of KMg4(PO4)3:Eu2+ at room temperature and a given temperature, respectively; A is a constant; k is the Boltzmann constant (8.617 × 10−5eV K−1). From above equation, the ΔE is calculated to be about 0.24eV (Figure 9).
The temperature-dependent of emission FWHM is related to the configuration coordinate model and the Boltzmann distribution and can be expressed by:18,29


where W0 is the FWHM at 0°C, hv represents the vibrational phonon energy, S means the Huang−Rhys parameter and k is the Boltzmann constant. With the temperature increase, the excited electrons spread to higher vibration levels and the radiative transitions from these different levels cause the emission band broadening.
Quantum efficiency and Electroluminescence properties of White-Light LED Lamp
Quantum efficiency of phosphors is another important technological parameter for practical application. The internal quantum efficiency (QE) of KMg4(PO4)3:0.01Eu2+ were measured and calculated by the following equations:30

in which Ls represents the luminescence emission spectrum of the sample; ER is the spectrum of the excitation light from the empty integrated sphere (without the sample); ES means the excitation spectrum for exciting the sample. As given in Figure 10, the internal QE of the KMg4(PO4)3:0.01Eu2+ phosphor is estimated to be about 50.44% under 365nm excitation. As a comparison, the internal QE of commercial BAM:Eu2+ phosphor is detected at the same condition and calculated to about 88.99%. The QE of KMg4(PO4)3:0.01Eu2+ can be further improved by optimization of the preparation conditions, because the QE depends closely on the prepared conditions, crystalline defects, particle size and morphology of the phosphor31,32.
To demonstrate the potential application of KMg4(PO4)3:Eu2+ phosphor, the electroluminescent spectrum of white LED lamp which was fabricated via using N-UV LED chips (λmax = 385nm) combing with blue-emitting KMg4(PO4)3:0.01Eu2+ phosphor, green-emitting (Ba,Sr)2SiO4:Eu2+ phosphor and red-emitting CaAlSiN3:Eu2+ phosphor was measured as given in Figure 11 with forward bias current of 2mA. The CIE color coordinates, correlated color temperature (CCT) and color rendering index (Ra) of this fabricated WLED lamp are determined to be (0.34, 0.35), 5227.08 and 96, respectively. The Ra was decided from the full set of the first eight CRIs shown in Table 2. The appropriate CCT value (5227.08) and high Ra value (96) demonstrate that the KMg4(PO4)3:Eu2+ can be a promising candidate for a blue-emitting phosphor for application of WLEDs.
Discussion
In conclusion, we report a systematic study on the preparation and crystal structure analysis of blue-emitting KMg4(PO4)3:Eu2+ phosphor and investigate their reflectance spectra, thermal stability, quantum efficiency and applications in N-UV LED. The phase composition and crystal structure of KMg4(PO4)3:Eu2+ were determined via the powder X-ray diffraction patterns and Rietveld refinement analysis. The optimal Eu2+ doping concentration in the KMg4(PO4)3 host is 1mol%. The critical energy transfer distance of this phosphor was calculated to be about 35.84Å and the concentration quenching mechanism is proved to be the dipole–dipole interaction. The investigation results also reveal that the as-prepared phosphor shows good thermal stability and the internal quantum efficiency is 50.44%. The white N-UV LEDs packaged by an N-UV chip with blue-emitting KMg4(PO4)3:Eu2+, green and red-emitting phosphors generate white light with high color rendering index (Ra = 96) and an appropriate correlated color temperature (5227.08K). These results demonstrate that KMg4(PO4)3:Eu2+ is a promising blue-emitting phosphor for N-UV LEDs.
Methods
Materials and Synthesis
A variety of blue-emitting KMg4(PO4)3:xEu2+ phosphors were prepared via a traditional high-temperature solid-state reaction. The constituent raw materials KH2PO4 (A. R.), MgO (A. R.), NH4H2PO4 (A. R.) and Eu2O3 (A. R.) were weighed in stoichiometric proportions and ground homogeneously in agate mortar. Firstly, the mixtures were preheated at 600°C for 2h in a muffle furnace in air to release NH3, CO2 and H2O. Then, the precursor was reground and heated at 1000°C for 6h in the thermal carbon reducing atmosphere (TCRA). Finally the furnace cooled to room temperature and the mixtures were ground in an agate mortar.
Materials Characterization
X-ray powder diffraction (XRD) patterns of the final products were identified on a D8 Advance diffractometer (Bruker Corporation, Germany) with Cu Kα radiation (λ = 0.15406nm) radiation. High quality XRD data for Rietveld refinement were collected by step scanning rate (8s per step with a step size of 0.02°) over a 2θ range from 5° to 100°. The photoluminescent excitation/emission (PLE/PL) spectra were detected by a Hitachi F-4600 fluorescence spectrophotometer (Japan) equipped using a150W Xe lamp as the excitation source. The temperature-dependent luminescence properties were measured on the same spectrophotometer which was assembled with a computer-controlled electric furnace and a self-made heating attachment. The diffuse reflectance spectra were obtained by a Varian Cary-5000 UV−vis−NIR spectrophotometer attached with an integral sphere. The room-temperature luminescence decay curves were obtained from a spectrofluorometer (Horiba, Jobin Yvon TBXPS) using a tunable pulse laser radiation (nano-LED) as the excitation. Quantum efficiency was measured by a fluoromax-4 spectrofluorometer (Horiba, Jobin Yvon) with an integral sphere at room temperature.
References
Bergh, A., Craford, G., Duggal, A. & Haitz, R. The Promise and Challenge of Solid-State Lighting. Phys. Today. 54, 42–47 (2001).
Brinkley, S. E. et al. Robust Thermal Performance of Sr2Si5N8:Eu2+: an Efficient Red Emitting Phosphor for Light Emitting Diode Based White Lighting. Appl. Phys. Lett. 99, 241106 (2011).
Krings, M., Montana, G., Dronskowski, R. & Wickleder, C. α-SrNCN:Eu2+- A Novel Efficient Orange-Emitting Phosphor. Chem. Mater. 23, 1694–1699 (2011).
Gwak, S. J., Arunkumar, P. & Im, W. B. A New Blue-Emitting Oxohalide Phosphor Sr4OCl6:Eu2+ for Thermally Stable, Efficient White-Light-Emitting Devices under Near-UV. J. Phys. Chem. C. 118, 2686–2692 (2014).
Chiu, Y. C. et al. Ca2PO4Cl:Eu2+: an Intense Near-Ultraviolet Converting Blue Phosphor For White Light-Emitting Diodes. J. Mater. Chem. 20, 1755–1758 (2010).
Li, H. F. et al. Sr1.7Zn0.3CeO4:Eu3+ Novel Red-Emitting Phosphors: Synthesis and Photoluminescence Properties. ACS Appl. Mater. Interfaces. 6, 3163–3169 (2014).
Xia, Z. G. & Liu, R. S. Tunable Blue-Green Color Emission and Energy Transfer of Ca2Al3O6F:Ce3+, Tb3+ Phosphors for Near-UV White LEDs. J. Phys. Chem. C. 116, 15604–15609 (2012).
Zhu, P. et al. Effect of SiO2 Coating on Photoluminescence and Thermal Stability BaMgAl10O17:Eu2+ under VUV and UV Excitation. Opt. Mater. 30, 930–934 (2008).
Im, W. B., Kim, Y.-I., Yoo, H. S. & Jeon, D. Y. Luminescent and Structural Properties of (Sr1−x, Bax)3MgSi2O8:Eu2+: Effects of Ba Content on the Eu2+ Site Preference for Thermal Stability. Inorg. Chem. 48, 557–564 (2008).
Dorenbos, P. Energy of the First 4f7-4f65d Transition of Eu2+ in Inorganic Compounds. J. Lumin. 104, 239–260 (2003).
Chen, J. et al. The Luminescence Properties of Novel α-Mg2Al4Si5O18:Eu2+ Phosphor Prepared in Air. Rsc Adv. 4, 18234–18239 (2014).
Tomaszewski, P. E., Maczka, M., Majchrowski, A., Waskowska, A. & Hanuza, J. Crystal Structure and Vibrational Properties of KMg4(PO4)3 . Solid State Sci. 7, 1201–1208 (2005).
Lan, X. F., W, Q. Q., Chen, Y. Y. & Tang, W. J. Luminescence Properties of Eu2+-Activated Kmg4(PO4)3 for Blue-Emitting Phosphor. Opt. Mater. 34, 1330–1332 (2012).
Chen, J., Liu, Y. G., Fang, M. H. & Huang, Z. H. Luminescence Properties and Energy Transfer of Eu/Mn-Coactivated Mg2Al4Si5O18 as a Potential Phosphor for White-Light LEDs. Inorg. Chem. 53, 11396–11403 (2014).
Piriou, B., Chen, Y. F. & Vilminot, S. Site-Selective Spectroscopy in Eu3+ Doped α-cordierite. Eur. J. Solid State Inorg. Chem. 35, 341–355 (1998).
Thim, G. P., Brito, H. F., Silva, S. A., Oliveira, M. A. & Felinto, M. C. Preparation and Optical Properties of Trivalent Europium Doped into Cordierite using The Sol–Gel Process. J. Solid State Chem. 171, 375–381 (2003).
Jiang, Z. Q. ., Wang, Y. H. & Wang, L. S. Enhanced Yellow-to-Orange Emission of Si-Doped Mg3Y2Ge3O12: Ce3+ Garnet Phosphors for Warm White Light-Emitting Diodes Sensors and Displays: Principles, Materials and Processing. J. Electrochem. Soc. 157, J155 (2010).
Liu, C. M. et al. High Light Yield of Sr8(Si4O12)Cl8:Eu2+ under X-ray Excitation and Its Temperature-Dependent Luminescence Characteristics. Chem. Mater. 26, 3709–3715 (2014).
Wang, D. Y., Huang, C. H., Wu, Y. C. & Chen, T. M. BaZrSi3O9:Eu2+: A Cyan-Emitting Phosphor with High Quantum Efficiency for White Light-Emitting Diodes. J. Mater. Chem. 21, 10818–10822 (2011).
Huang, C. H. & Chen, T. M. A Novel Single-Composition Trichromatic White-Light Ca3Y(GaO)3(BO3)4:Ce3+, Mn2+, Tb3+ Phosphor for UV-Light Emitting Diodes. J. Phys. Chem. C. 115, 2349–2355 (2011).
Im, W. B. et al. Sr2.975−x BaxCe0. 025AlO4F: A Highly Efficient Green-Emitting Oxyfluoride Phosphor for Solid State White Lighting. Chem. Mater. 22, 2842–2849 (2010).
Blasse, G. Energy Transfer in Oxidic Phosphors. Philips Res. Rep. 24, 131 (1969).
Dexter, D. L. A Theory of Sensitized Luminescence in Solids. J. Chem. Phys. 21, 836–850 (1953).
Blasse, G. Energy Transfer in Oxidic Phosphors. Phys. Lett. 28, 444–445 (1968).
Xie, R. J., Hirosaki, N., Kimura, N., Sakuma, K. & Mitomo, M. 2-Phosphor-Converted White Light-Emitting Diodes using Oxynitride/Nitride Phosphors. Appl. Phys. Lett. 90, 191101–191101-3 (2007).
Geng, D. L. et al. Color Tuning via Energy Transfer in Sr3In(PO4)3:Ce3+/Tb3+/Mn2+ Phosphors. J. Mater. Chem. 22, 14262–14271 (2012).
Lv, W. Z. et al. Synthesis, Structure and Luminescence Properties of K2Ba7Si16O40:Eu2+ for White Light Emitting Diodes. J. Phys. Chem. C. 118, 4649–4655 (2014).
Huang, C. H., Liu, W. R. & Chen, T. M. Single-Phased White-Light Phosphors Ca9Gd (PO4)7:Eu2+, Mn2+ under Near-Ultraviolet Excitation. J. Phys. Chem. C. 114, 18698–18701 (2010).
Henderson, B. & Imbusch, G. F. Optical Spectroscopy of Inorganic Solids. Optical Spectroscopy of Inorganic Solids Clarendon Press, Oxford, U.K., 1989.
Xu, Y. S. et al. Efficient Near-Infrared Down-Conversion in Pr3+–Yb3+ Codoped Glasses and Glass Ceramics Containing LaF3 Nanocrystals. J. Phys. Chem. C. 115, 13056–13062 (2011).
Dexter, D. X. & Schulman, J. H. Theory of Concentration Quenching in Inorganic Phosphors. J. Chem. Phys. 22, 1063–1070 (1954).
Bachmann, V., Ronda, C., Oeckler, O., Schnick, W. & Meijerink, A. Color Point Tuning for (Sr, Ca, Ba)Si2O2N2:Eu2+ for White Light LEDs. Chem. Mater. 21, 316–325 (2009).
Acknowledgements
This work was sponsored by National Natural Science Foundation of China (Grant No. 51472223), the Program for New Century Excellent Talents in University of Ministry of Education of China (Grant No. NCET-12-0951).
Author information
Authors and Affiliations
Contributions
Y.G.L. and J.C. conceived the project. J.C. and H.K.L. designed and performed the experiments. J.C., L.F.M. and M.H.F. analyzed the data. J.C. and Z.H.H. wrote the manuscript. All the authors discussed the results and commented on the manuscript at all stages.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article's Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder in order to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Chen, J., Liu, Y., Mei, L. et al. Crystal structure and Temperature-Dependent Luminescence Characteristics of KMg4(PO4)3:Eu2+ phosphor for White Light-emitting diodes. Sci Rep 5, 9673 (2015). https://doi.org/10.1038/srep09673
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep09673
This article is cited by
-
Exploring the orange–red emission from novel vanadate-based nanomaterials for highly innovative photonic applications
Bulletin of Materials Science (2023)
-
Sr3Gd(PO4)3: Dy3+ phosphors for lighting applications
Journal of Sol-Gel Science and Technology (2023)
-
Structural and Optoelectronic Investigation of Combustion-Derived Ba2Zn2La4O10: Er3+ Green Emitters for n-UV-Based White LEDs
Journal of Electronic Materials (2022)
-
Design of color tunable SrLa2Al2O7:Eu3+ perovskite type nanophosphor for near-ultraviolet excited white LEDs
Journal of Materials Science: Materials in Electronics (2022)
-
Ba2Zn2La4O10:Sm3+: A novel orange–red emitting nanophosphor with high color purity for WLEDs applications
Journal of Materials Science: Materials in Electronics (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.