Abstract
In this study, we collected life table data for the sweetpotato weevil, Cylas formicarius, grown on Ipomoea batatas and Ipomoea triloba and analyzed them using an age-stage, two-sex life table. We also demonstrated the growth potential of C. formicarius on these two host plants by using population projection. These data will be useful to the growers to the selection or eradication of host plants in an integrated control strategy for C. formicarius for the entire area of the targeted areas. We found that C. formicarius developed faster on I. batatas than on I. triloba. The developmental times of the larval and pupal stages on I. batatas than on I. triloba were 37.01 and 8.3 days. The adult females emerged before and began to produce eggs at 42 days earlier when reared on I. batatas. The fecundity of females was 90.0 eggs on I. batatas significantly higher than the mean fecundity of 68.5 eggs on I. triloba. Although this insect has a higher intrinsic rate of increase on I. batatas, the study indicated that C. formicarius can successfully survive and reproduce on both host plants.
Similar content being viewed by others
Introduction
A life table is a convenient and comprehensive method for summarizing the survival and reproductive potential of a population. Using a life table, one can compare the growth potential of an insect on different host plants, under different environmental conditions1,2,3. Because the age-stage, two-sex life table can describe stage differentiation and include both sexes, it can precisely reveal the actual life history of the insect species and life table have been widely applied in the study of various ecological aspects of interest in relation to insect pests and their natural enemies4. Understanding the demography of an insect under variable conditions is the cornerstone for developing an environmentally friendly pest management strategy.
The sweetpotato weevil, Cylas formicarius (F.) (Coleoptera: Brentidae), is the most serious pest of sweet potato (Ipomoea batatas [L.] Lam., Convolvulaceae)5 both in the field and in storage6. In the Marianas Islands, Ipomoea triloba L. (Convolvulaceae) (littlebell, or Aiea morning glory) is widespread and serves as an alternative host for C. formicarius. This vine is reported to grow around sweet potato fields and has been recorded as an alternate host of another pest of sweet potato Euscepes postfasciatus (Fairmaire) (Coleoptera: Brentidae)7. In addition to I. batatas, the major host plant of C. formicarius5, at least 49 other members of the Convolvulaceae have been recorded as hosts for C. formicarius, which has been recorded feeding on seven genera in six tribes within this plant family8,9. In Guam and other Micronesian Islands, I. triloba is widespread and serves as an alternative host for C. formicarius10. Because of the cryptic nature of the larvae and the nocturnal activity of the C. formicarius adults, it is difficult to control this pest using chemicals11. In addition, the understanding the life history of C. formicarius will help and makes the pest easiest to control with long-residual pesticides that are now out of favor and often unavailable5.
To systematically understand the role of main and alternative hosts in the population explosion of C. formicarius, information on its demography, including development, survival and fecundity on each host plant, is needed. In this study, we measured these life table parameters of C. formicarius on I. batatas and I. triloba and analyzed them using an age-stage, two-sex life table. Furthermore, we estimated the potential rate of growth of C. formicarius on each of these two host plants using a population projection. If C. formicarius can survive on I. triloba, growers have to focus in controlling pest incidence on the alternative host as well. These data will be useful in the selection or eradication of host plants for an integrated control strategy for C. formicarius.
Materials and methods
The experiments were conducted from January 2013 to March 2014 in a shade house at the Western Pacific Quarantine Biocontrol Laboratory of the University of Guam (13.43 °N, 144.80 °E and 54.3 m).
Study area
The shade house was 100 m2 in area; the walls and roof were constructed of shade cloth. Mean temperature in the shade house was 31 °C (range 29.5–32.5 °C), mean relative humidity was 78% (range 76–80%) and the natural photoperiod was 14–16:10-8 (L:D) h.
Plants
Sweet potato (I. batatas) and Aiea morning-glory (I. triloba) cuttings (ca. 30 cm) were planted in individual pots (20 cm diameter × 30 cm deep) filled with disease- and weed-free Miracle Grow potting soil. One hundred and fifty such pots were planted with each of the two plant species for use in the experiments. Fertilizer in the form of Nitrogen, Phosphorus, Potassium and Sulfur was applied at the actual time of planting according to published recommendations12.
Insects
Pheromone lures consisting of rubber septa loaded with Z3-dodecenyl-E2-butenoate, sealed in an impermeable bag for shipping and storage, were obtained from Chem Tica Internacional S.A. (San José, Costa Rica)13. Pherocon unitraps (Trécé Incorporated, Adair, Oklahoma, USA) baited with these lures were used to trap adult C. formicarius in sweet potato fields in Latte Heights (Guam, USA) during 201014. The trapped adults were taken to the laboratory, placed in batches in collapsible cages (12 × 10 × 10 cm), fed leaves and pieces of the sweet potato and maintained at 22 ± 2 °C, 70–80% relative humidity and a 16:8 h L:D photoperiod15. In this colony, 5-6 generations were completed before the offspring were used for these experiments. For all experiments, 3-4 week-old adults were obtained from these laboratory colonies16.
Experiments
Each pupae were taken out of the insect colony and placed in an individual test tube. Upon emergence, fifty pairs of adults for each plant species consisting of virgin males and females were taken; each female and male was placed on the both potted plants. Enlarged stalks and woody plants of (comparable sizes of I. batatas and I. triloba) were chosen whenever possible, as females prefer to deposit eggs on these plants. The plants were covered with collapsible rearing cages (30 × 30 × 60 cm) to prevent escape. Eggs were deposited singly, in cavities or cells formed by the adult in roots and vines of the host plant5. The egg is creamy white, broadly oval and narrowed at the attached end. The egg becomes darker just before hatching and the dark head of the larva becomes noticeable17. Data were taken daily on the number of eggs laid, the number hatched and the duration of each developmental stage of C. formicarius on the two host plants. For the life table studies a total of 150 eggs, all laid within 24 h, were collected from the 50 pairs for both I. batatas and I. triloba. The eggs were checked daily until they hatched and then checked the larvae daily for survival. Since immature stages of the C. formicarius grow inside host plant, the stems have been taken out of the cages observed the immatures’ growth and survival. Daily counts of surviving larvae at each stage as well as number of eggs laid by each female were recorded.
Statistical analysis
Raw data of daily development and reproduction of each individual were analyzed according to the age-stage, two-sex life table theory18 as described by Chi2. The population parameters calculated were the age-stage survival rate (sxj, the probability that a newly laid egg will survive to age x and stage j), the age-specific fecundity of female (fxj, the mean fecundity of females at age x), the age-specific survival rate (lx, the probability of a newly laid egg survives to age x) and the age-specific fecundity (mx, the mean fecundity of individuals at age x). In the age-stage, two-sex life table, the lx and mx are calculated as


where m is the number of stages.
The net reproductive rate (R0) is calculated as

The intrinsic rate of increase (r) is estimated by using iterative bisection method from

with age x indexed from 019. The finite rate of increase (λ) is calculated as λ = er. The mean generation time is defined as the length of time that a population needs to increase to R0-fold of its size (i.e., erT = R0 or λT = R0) at the stable age-stage distribution and is calculated as

The life expectancy (exj) of individuals at age x and stage j is calculated according to Chi and Su4, while the reproductive value (vxj) is calculated according to Tuan et al20. The means and standard errors of the life table parameters were estimated by using the bootstrap procedure21 with bootstrap number m = 40,000 to ensure more precise estimates. TWOSEX-MSChart Visual BASIC (version 6, service pack 6) for Windows (available at http://140.120.197.173/Ecology/ (Chung Hsing University) and http://nhsbig.inhs.uiuc.edu.tw/www/chi.html (Illinois Natural History Survey) were used to analyse our age-stage, two-sex life table data (Chi, H. TWOSEX-MSChart: a computer program for the age-stage, two-sex life table analysis. (http://140.20.197.173/Ecology/Download/Twosex-MSChart.rar, 2013)
The paired bootstrap test21,22 was used to compare the differences in developmental time, adult longevity, adult preoviposition period (APOP), total preoviposition period (TPOP), oviposition days and fecundity between treatments. The population parameters (r, λ, R0 and T) between two treatments were also compared by using the paired bootstrap test based on the confidence interval of differences21,22.
Population projection
Survival rate and fecundity data were used to project population growth according to Chi and Liu18 and Chi23. The computer program TIMING-MSChart (Chi, H. TWOSEX-MSChart: a computer program for age-stage, two-sex life table analysis, http://140.120.197.173/Ecology/, 2014) used in this projection is also available at the above-mentioned web sites.
Results
Out of 150 eggs (N) used at the beginning of life table study on I. batatas, 149 eggs hatched and 146 larvae successfully developed to adult stage (Table 1). We found that larvae and pupae of C. formicarius developed faster on I. batatas than on I. triloba. The developmental times of the larval and pupal stages were 37.0 and 8.3 days, respectively, when reared on I. batatas, which were significantly shorter than those observed on I. triloba (54.9 and 16.9 days, respectively) (Table 1). Adult females emerged earlier and began to produce eggs at 42 days earlier when reared on I. batatas. There was no difference between the adult preoviposition periods (APOP) reared on I. batatas and I. triloba (P = 0.5234). The mean of total preoviposition period (TPOP) on I. batatas was 50.1 d. When reared on I. triloba, the first female adult emerged much later (day 60) and the mean TPOP was 77.2 d, significantly later than on I. batatas. The mean fecundity (F) of females was 90.9 eggs on I. batatas, significantly higher than the mean fecundity of 68.57 eggs on I. triloba (Table 1). Out of 150 eggs (N) used at the beginning of life table study on I. batatas, 90 individuals emerged as female adults (Nf); meanwhile, the values of F, Nf and N on I. triloba were 68.57, 83 and 150, respectively.
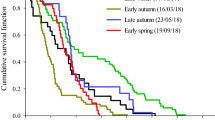
The faster development on I. batatas could also be observed in the age-stage survival rate (sxj). The curves of females and males emerged at 42 and 39 d, respectively, for I. batatas (Fig. 1A); in contrast, female and male adults appeared at 60 and 64 d, respectively, on I. triloba (Fig. 1B). The female age-specific fecundity (fxj) and age-specific fecundity (mx) on I. batatas not only began much earlier (at 42 d) than that on I. triloba (at 60 d) (Fig. 2), but were also significantly higher than for the latter.
The intrinsic rate of increase (r), the finite rate (λ) and the net reproductive rate (R0) of the sweetpotato weevil reared on I. batatas were 0.0622 d−1, 1.0663 d−1 and 54.55 eggs, respectively, all significantly higher (P = 0.0001) than the values obtained on I. triloba (0.0418 d−1, 1.0426 d−1 and 37.94 eggs, respectively) (Table 2). On the other hand, the mean generation time (T) obtained on I. batatas (62.26 d) was shorter than that on I. triloba (87.07 d).
The longevity of the C. formcarius at age zero (e01) was 135.63 d on I. batatas, which did not differ significantly from the longevity of 134.68 d on I. triloba (Fig. 3). These were exactly the mean longevity of all individuals used in the life table study. At age zero, the reproductive values (v01) were exactly the same as the finite rates on both host plants, i.e., 1.0663 d−1 on I. batatas and 1.0426 d−1 on I. triloba (Fig. 4). The value of vxj on I. batatas jumped to 45.5 d−1 at 42 d when female adults emerged; when reared on I. triloba, the vxj value jumped to 54.93 d−1 when female emerged later at 60 d.
The population projection suggested that C. formicarius will grow much faster on I. batatas than on I. triloba (Fig. 5). Beginning with 150 eggs, the population would undergo four generations and the total population would exceed five million on I. batatas after 180 d, while the weevils would go through only three generation on I. troloba, for a final size of approximately 124,000.
Discussion
The main host of C. formicarius and all species of sweetpotato weevil (Euscepes postfasciatus (Fairmaire), Daealus tuberosus (Zimmerman) and Cylas puncticollis (Boheman) is the sweet potato24,10,25. Another species, Cylas brunneus (Fabricius) has found only on sweet potatoes. Carrot (Daucus carota (Hoffm.) Schübl. & G. Martens), radish (Raphanus sativus L.) and morning glory (I. triloba) are known to serve as additional hosts for C. formicarius24. Since I. triloba is widespread in the Pacific Islands and other regions of the world15, it is one of the main alternative hosts for C. formicarius.
Because the age-stage, two-sex life table takes the variable developmental rate among individuals into consideration, the overlapping between stages can be observed in the curves of sxj. However, if the same data were analyzed using the traditional female age-specific life table, such as Lewis-Leslie matrix26,27,28,29, the stage differentiation would not be revealed. The age-specific survival rate (lx) (Fig. 2) is the simplified version of the age-stage survival rate (Fig. 1). Although the stage differentiation and overlapping could not be observed in the lx curve, it is nevertheless constructed by using the age-stage, two-sex life table and could correctly describe the change of the survival rate with age. Huang and Chi30 demonstrated that an erroneous lx curve would be obtained if the traditional female age-specific were used.
Chi2 proved mathematically the relationship among F, Nf, N and R0 as R0 = F × (Nf /N). In this study, the values of F, Nf, N and R0 on both host plants are completely consistent with the proven relationship. This relationship can be used to detect errors in life table analysis.
Although the weevil could survive longer than 160 days on both host plants (Fig. 1), the reproductive values (Fig. 4) showed that female adults did not contribute to the population growth after 92 d when reared on I. batatas, while C. formicarius on I. triloba did not have any contribution to population growth after 116 d. It seems that females from I. triloba can migrate to nearby I. batatas field after 92 d and still produce offspring.
Many plants function as oviposition sites for most herbivorous insects which deposit their eggs on all parts of the plants31. Leaf boundary layer effects both insect egg deposition behavior and progress of the embryo inside the egg31,32. The effects of eggs on plants consist of egg-induced changes of photosynthetic activity and of the plant’s secondary metabolism31. In this study, out of 150 eggs, four eggs did not hatched. This could be possibly due to ovicidal effect of host plants of I. batatas or I. triloba32,33.
A population projection based on an age-stage, two-sex life table can reveal the change of stage structure during population growth. Understanding stage structure is important to pest management because the dispersal and damage capability of insects varies with stage. This study demonstrates that such a life table can provide a comprehensive description of the fitness of an insect population on a given host plant. This study provides an interesting information on life table comparison of a C. formicarius on two of its host species. This shows that implications of the life-table data in IPM and/or weevil biology. The measure of the degree of dispersal from morning glory to sweet potato is clear, therefore the morning glory management around sweet potato fields is warranted. However, further studies are required on some insects occurring in alternate host plants may have to be proven to belong to genetically distinct populations or even cryptic species that do not cause much damage to crops of interest. Additional studies are also required if I. triloba is also the alternative host of another pest of sweet potato (i.e., Euscepes postfasciatus) an IPM strategy to manage I. triloba needs to consider E. postfasciatus in the picture.
This study determined that the C. formicarius can survive, on average, longer than four months on both host plants and successfully produce offspring for a month. Because I. triloba can be used successfully by C. formicarius as an alternate host, we suggest that growers should include the clearing of I. triloba from around sweet potato fields and storage facilities in their management program.
Additional Information
How to cite this article: Reddy, G. V. P. and Chi, H. Demographic comparison of sweetpotato weevil reared on a major host, Ipomoea batatas and an alternative host, I. triloba. Sci. Rep. 5, 11871; doi: 10.1038/srep11871 (2015).
References
Morris, R. F. & Miller, C. A. The development of life tables for the spruce budworm. Can. J. Zool. 32, 283–301 (1954).
Chi, H. Life-table analysis incorporating both sexes and variable development rates among individuals. Environ. Entomol. 17, 26–34 (1988).
Yin, J. et al. Effects of elevated CO2 associated with maize on multiple generations of the cotton bollworm, Helicoverpa armigera. Entomol. Exp. Appl. 136, 12–20 (2010).
Chi, H. & Su, H. Y. Age-stage, two-sex life tables of Aphidius gifuensis (Ashmead) (Hymenoptera: Braconidae) and its host Myzus persicae (Sulzer) (Homoptera: Aphididae) with mathematical proof of the relationship between female fecundity and the net reproductive rate. Environ. Entomol. 35, 10–21 (2006).
Chalfant, R. B. et al. Ecology and management of sweet potato insects. Annu. Rev. Entomol. 35, 157–180 (1990).
Sherman, M. & Tamashiro, M. Sweet potato weevils in Hawaii and their biology and control 38p. (Hawaii Agricultural Experimental Station, Hawaii, 1954).
Hill, D. Agricultural Insect Pests of the Tropics & Their Control 516p (University of Cambridge University Press, Cambridge, UK, 1983).
Pemberton, C. E. A note. Proc. Hawaiian Entomol. Soc. 12, 9 (1943).
Austin, D. F. et al. Convolvulaceae and Cylas: a proposed hypothesis on the origins of this plant/insect relationship. Trop. Agric. 68, 162–170 (1991).
Reddy, G. V. P. et al. Estimation of the population density of the sweet potato weevils on the Mariana Islands. J. Entomol. Acarol. Res. 44, 18–21 (2012b).
Reddy, G. V. P. et al. Efficacy of pheromone trapping of the sweet potato weevil, Cylas formicarius (Coleoptera: Brentidae): based on dose, septum age and attractive radius. Environ. Entomol. 43, 767–773 (2014a)
Nandawani, D. & Tudela, A. Sweet Potato in the CNMI. 28p (Northern Marianas College Publication # 02/2010, 2010).
Reddy, G. V. P. et al. Efficient sex pheromone trapping: catching the sweet potato weevil Cylas formicarius. J. Chem. Ecol. 38, 846–853 (2012a).
Reddy, G. V. P. et al. Laboratory and field efficacy of entomopathogenic fungi for the management of the sweetpotato weevil, Cylas formicarius (Coleoptera: Brentidae). J. Inverte. Pathol. 122, 10–15 (2014b).
Leng, P. H. & Reddy, G. V. P. Bioactivity of selected eco-friendly pesticides against the sweet potato weevil, Cylas formicarius (Fabricius) (Coleoptera: Brentidae). Florida Entomol. 95, 1040–1047 (2012).
Gadi, N. & Reddy, G. V. P. Are sweet potato weevils differentially attracted to certain colors? Ann. Entomol. Soc. Am. 106, 274–278 (2014).
Cockerham, K. L. et al. The biology of the sweet potato weevil. Louisiana Agricultural Experiment Station Technical Bulletin 483, 30 (1954).
Chi, H. & Liu, H. Two new methods for the study of insect population ecology. Bull. Inst. Zool. Acad. Sin. 24, 225–240 (1985).
Goodman, D. Optimal life histories, optimal notation and the value of reproductive value. Am. Natur. 119, 803–823 (1982).
Tuan, S. J. et al. Effects of green manure cover crops on Spodoptera litura (Lepidoptera: Noctuidae) populations. J. Econ. Entomol. 107, 897–905 (2014).
Efron, B. & Tibshirani, R. J. An Introduction to the Bootstrap (Chapman and Hall, New York, 1993).
Polat Akköprü, et al. Demographic assessment of plant cultivar resistance to insect pests: a case study of the dusky-veined walnut aphid (Hemiptera: Callaphididae) on five walnut cultivars. J. Econ. Entomol 108, 378–387 (2015).
Chi, H. Timing of control based on the stage structure of pest populations: a simulation approach. J. Econ. Entomol. 83, 1143–1150 (1990).
Muruvanda, et al. Additional alternate hosts of the Sweet potato weevils Cylas formicarius elegantulus and Euscepes postfasciatus (Coleoptera: Curculionidae) in Hawaii. Proc. Hawaiian Entomol. Soc. 26, 93–96 (1986).
Loebenstein, G. & Thottappilly, G. (Eds.). The Sweetpotato 522 p (Springer Science, 2009).
Lewis, E. G. On the generation and growth of a population. Sankhya 6, 93–96 (1942).
Leslie, P. H. On the use of matrices in certain population mathematics. Biometrika 33, 183–212 (1945).
Birch, L. C. The intrinsic rate of natural increase in an insect population. J. Anim. Ecol. 17, 15–26 (1948).
Carey, J. R. Applied Demography for Biologists with Special Emphasis on Insects. (Oxford University Press, New York, 1993).
Huang, Y. B. & Chi, H. Age-stage, two-sex life tables of Bactrocera cucurbitae (Coquillett) (Diptera: Tephritidae) with a discussion on the problem of applying female age-specific life tables to insect populations. Insect Sci. 19, 263–273 (2012).
Hilker, M. & Meiners, T. Plants and insect eggs: How do they affect each other? Phytochemistry 72, 1612–1623 (2011).
Seino, Y. et al. An ovicidal substance produced by rice plants in response to oviposition by the whitebacked planthopper, Sogatella furcifera (Horváth) (Homoptera: Dephacidae). Appl. Entomol. Zool. 31, 467–473 (1996).
Braga, Y. F. B. et al. Insecticidal activity of 2-tridecanone against the cowpea weevil Callosobruchus maculatus (Coleoptera: Bruchidae). An. Acad. Bra. Ciências 79, 35–39 (2007).
Acknowledgements
This project was supported by the FY 2011 Pacific Islands Area Conservation Innovation Grants (PIA-CIG) Program, Grant Agreement No. 69-9251-11-902 and the Natural Resources Conservation Service (NRCS)-USDA. The USDA is an equal opportunity provider and employer.
Author information
Authors and Affiliations
Contributions
G.V.P.R. and H.C. wrote the main manuscript text and H.C. prepared figures 1–5. All authors reviewed the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Reddy, G., Chi, H. Demographic comparison of sweetpotato weevil reared on a major host, Ipomoea batatas, and an alternative host, I. triloba. Sci Rep 5, 11871 (2015). https://doi.org/10.1038/srep11871
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep11871
This article is cited by
-
Tailoring IPM plans to fight a cloaked pest: helping smallholder farmers combat the sweetpotato weevil in sub-Saharan Africa
CABI Agriculture and Bioscience (2024)
-
The effect of spermine on Tetranychus urticae-Cucumis sativus interaction
BMC Plant Biology (2023)
-
Temperature-dependent development and reproduction of Tarsonemus confusus (Acari: Tarsonemidae): an important pest mite of horticulture
Experimental and Applied Acarology (2022)
-
Diets for Tamarixia triozae adults before releasing in augmentative biological control
BioControl (2022)
-
Effect of temperature for mass rearing of Spilosoma obliqua on an artificial diet using age-stage, two-sex life table approach
Biologia (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.