Abstract
To fabricate high-performance metal-halide perovskite solar cells, a thermal annealing process is indispensable in preparing high quality perovskite film. And usually such annealing is performed on hot plate. However hot-plate annealing could cause problems such as inhomogeneous heating (induced by non-tight contact between the sample and the plate), it is also not fit for large scale manufactory. In this paper, we conduct the annealing process in air-heated oven under various humidity environments, and compared the resulted films (CH3NH3PbI3−xClx) and devices (Al/PC61BM/CH3NH3PbI3−xClx/PEDOT:PSS/ITO/glass) with that obtained via hot-plate annealing. It is found that the air-heated-oven annealing is superior to the hot-plate annealing: the annealing time is shorter, the films are more uniform, and the devices exhibit higher power conversion efficiency and better uniformity. The highest efficiencies achieved for the oven and hot-plate annealing processes are 14.9% and 13.5%, and the corresponding standard deviations are 0.5% and 0.8%, respectively. Our work here indicates that air-heated-oven annealing could be a more reliable and more efficient way for both lab research and large-scale production.
Similar content being viewed by others
Introduction
Nowadays many scientists and engineers worldwide are working on development of photovoltaic devices, which are promising in providing clean energy for human beings in the future. Metal-halide perovskite solar cells have recently emerged at the forefront of such photovoltaic research, and in a very short time of about 5 years, their power conversion efficiencies have skyrocketed from about 3% to higher than 20%1,2,3,4,5,6,7. To fabricate high-performance perovskite solar cells, the most crucial step is to form high quality crystallinity perovskite film (dense, pure, uniform), for which a thermal annealing process is indispensable no matter how the initial film is formed (vapor-deposited process8,9,10, one-step solution process11,12,13,14, two-step depositing3,15,16, solution-engineering5,6,17, printing approach18,19,20,21,22 and solution-annealing postprocessing23,24). Usually the annealing is done on hot plate. However, annealing on hot plate is not suitable for large scale production and could have drawbacks that are difficult to overcome such as inhomogeneous heating induced by non-tight contact between the sample and the plate. Doing the annealing in air-heated oven could be a better approach. However, it has been seldom employed25,26, not to mention detailed investigation.
Here we do a brief analysis and comparison between the two approaches. In case of hot-plate annealing, the bottom of the sample directly contacts the surface of the plate, and the other side of the sample directly exposed to air (or nitrogen gas), the air nearby the surface could be heated hot, but the air temperature should gradually decrease away from the sample surface. Hence it is reasonable to state that the temperature must have certain gradients from the very bottom of the sample to the surface of the sample to the air environment. So the whole system (sample and the air environment) is not uniform in temperature. Whereas in case of air-heated-oven annealing, hot air with well-controlled temperature is filled into the oven and heated everything inside the oven, and hence the whole system is uniform in temperature. Also for the hot-plate annealing, the sample must closely contact the surface of the hot plate, which requires very flat surfaces for both the hot plate and the bottom of the sample. This sometimes is quite difficult to satisfy since small particles and other contaminations all can damage the flatness of these surfaces. Hence uniform heating for even one sample could somehow be hard to guarantee, not to mention uniform heating for different samples annealed at different batches. Such problem could seriously damage the performance uniformity of the annealed films and their corresponding devices. And in most cases one cannot put many samples on a hot plate at one time. These problems can all be avoided in air-heated oven. The samples can be put in any positions and in any postures in the oven, making the annealing process much more convenient and controllable, which is very suitable for large-scale production.
In this study, PbCl2 + 3CH3NH3I precursor films on PEDOT:PSS/ITO/glass substrates were annealed both on hot plate and in air-heated oven under various humidity (It is reported that suitable humidity during the film annealing can benefit the device performance due to formation of better-morphology films27,28,29,30,31,32,33,34). The annealing process is to release the redundant organic halide CH3NH3Cl or other unknown species35,36 and finally form the CH3NH3PbI3−xClx perovskite films (seen in Fig. 1). We found that the oven annealing results in more uniform CH3NH3PbI3−xClx films with fewer pin-holes, the corresponding perovskite solar cells also exhibit superior performances in efficiency and uniformity. Meanwhile, we systematically investigated the humidity effects on the film morphology and the device performance.
Results and Discussion
Figure 2 gives the Scanning Electron Microscopy (SEM) of the P films (films annealed on the hot plate) and the O films (films annealed in the oven) in different humidity environments. It is seen that for the P films, there are many small pin-holes under a relative humidity (RH) of 10%, and there are less and less pin-holes with the RH increasing to 20% and then 30%, and their sizes becomes smaller and smaller. The tide reverses when the RH is further increased to 40%: more pin-holes appear and their sizes increase. Meanwhile, relative large crystals start to form and the film seemingly loses its continuity. The tendency continues with increasing the RH to 50%, larger crystals form and the film breaks in certain areas. At 60% RH, it can be clearly seen that the film actually really breaks in most areas, and even larger crystals form with well-discerned gaps between them. With further increasing the humidity (70% to 90% RH), larger and larger crystals form with bigger and bigger gaps between them, and the film completely breaks in all the areas. For the O films, the overall tendency of the film evolution with humidity is the same. In comparison, the O film is more uniform than the P film for all the humidity. Under 10% to 40% RH, the O films are all very uniform with much fewer and smaller pin-holes, especially for the case of 30% RH, the film is very uniform and almost pin-hole free. Even at 40% RH (under which the P film already starts to break), the film is clearly continuous with only very small pin-holes. Even for the crystals formed at high humidity levels (50% to 90% RH), they are much more uniform in size for the O films. From these images it can also be concluded that the humidity has great influence over the formation of the perovskite film during the annealing process. And it is clear that for both the O films and the P films, a RH of 30% is optimal.
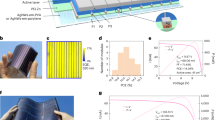
Photovoltaic devices were then fabricated based on the P films and the O films. The devices were structured as Al/PC61BM/CH3NH3PbI3−xClx/PEDOT:PSS/ITO/glass (shown in Fig. 3a). The energy level diagram of the device is displayed in Fig. 3b. The cross sectional SEM image of one O device (with the film formed at 30% RH) is given in Fig. 3c, in which each layer can be clearly discerned. And the thickness of CH3NH3PbI3−xClx film is about 260 nm. We found for both the O devices and the P devices, the best performance was observed for the film formed under 30% RH. J-V curves of the two best devices are given in Fig. 3d. The P device shows a PCE of 13.5%: VOC = 0.98 V, JSC = 20.1 mA/cm2, and FF = 0.69. The O device exhibits a PCE of 14.9%: VOC = 1.02 V, JSC = 20.3 mA/cm2, FF = 0.72. It is seen that VOC, JSC and FF are all improved by air-heated-oven annealing, and as a result an over 10% improvement in PCE is achieved. EQE of the two best devices are presented in Fig. 3e, together with the integrated current density as a function of wavelength37. The integrated JSC is 19.5 and 19.2 mA/cm2 for the O device and the P device, respectively. They are both slightly smaller than the measured ones from the J-V curves. This could be caused by device degradation during their exposure in air (during the transfer and EQE test process). The EQE spectrum show the onset of photocurrent at 790 nm, consistent with the reported band gap of CH3NH3PbI3−xClx25.
(a) Device structure of the perovskite inverted planar devices. (b) Energy level diagram of the device. (c) Cross-sectional SEM image of the device. (d) J-V curves of the best devices tested under 1.5 AM at 100 mW/cm2 illumination (scan rate: 200 mV/s; RP/RO: reverse scan of the P/O device; FP/FO: forward scan of the P/O devices). (e) EQE of the best devices. The integrated JSC are 19.5 mA/cm2 and 19.2 mA/cm2 for the O device and the P device, respectively.
To investigate uniformity of the device performance, 40 O devices/40 P devices from 3 batches are used to do the statistic study for each humidity condition. Average values of the device parameters (including JSC, VOC, FF and PCE) are summarized in Table 1, and are plotted in Fig. 4 as circles and squares. It is seen that overall for both the O devices and the P devices, the average VOC, JSC, FF and PCE all increase with the humidity in the range of 10% to 30% RH, and then drop with further increasing the humidity (30% to 90% RH). In comparison, the O device almost always shows superior VOC/JSC/FF/PCE over the P device under each humidity condition. Backing to Fig. 2, it can be easily found: 1) the dependence of the performance and its uniformity on the humidity is basically consistent with uniformity of the film morphology: the more uniform the film, the better the device performance as well as the performance uniformity. 2) The dependence of the performance and its uniformity on the annealing approach is also fundamentally consistent with uniformity of the film morphology: the more uniform the film, the better the device performance as well as the performance uniformity. Surprisingly, at very high humidity of 90% RH, the device still shows a PCE of around 5%. As can be imaged, the large gap area between the crystals might be covered by a CH3NH3PbI3−xClx thin film, which together with the PC61BM layer could prevent possible large current leakage between the anode and the cathode, and hence the device still can maintain a normal behavior. However, light current generated in the gap area should be very small and dark current in these areas should be quite large. Thereby these gap areas play a negative role of dragging down the overall light current density of the device and pushing up the overall dark current density of the device, and as a result, leading to smaller JSC, VOC, and FF.
In Table 1, the standard deviation values of the device parameters were also provided. It is seen that for all the humidity, the O devices exhibit better uniformity indicated by the smaller standard deviations of all the parameters (JSC, VOC, FF and hence PCE). As a representative, histograms of the statistic are shown in Fig. 5 for the O devices and the P devices under relative humidity 30%. As can be clearly seen, the O devices have much better performance and much better uniformity for all the parameters. For example, the PCE varies in a range of 12.4% to 14.9% with a deviation of 0.5%, whereas that of the P device is 11.3% to 13.5% with a deviation of 0.8%. The FF shows even more remarkable difference: 68% to 73% with a deviation of ~1.3% vs 63% to 71% with a deviation of 2.6%. Here we list the minimum and maximum values of the device parameters and their corresponding standard deviation Δ for the O/P devices. For the O devices, VOC: 0.945 − 1.022 V, Δ = 0.024 V; JSC: 18.1 − 20.5 mA/cm2, Δ = 0.8 mA/cm2; and FF: 0.68 − 0.73, Δ = 0.01; PCE: 12.5% – 14.9%, Δ = 0.6%; for the P devices, VOC: 0.901 – 1.000 V, Δ = 0.03 V; JSC: 17.4 – 20.3 mA/cm2, Δ = 0.9 mA/cm2; FF: 0.63 – 0.71, Δ = 0.03; PCE: 10.4% – 13.5%, Δ = 0.8%.
Conclusions
In summary, air-heated-oven annealing is employed in preparing CH3NH3PbI3−xClx films for high-performance perovskite solar cells (Al/PC61BM/CH3NH3PbI3−xClx/PEDOT:PSS/ITO/glass). The resulted CH3NH3PbI3−xClx films under a wide range of humidity (10% to 90% RH) are more uniform than the corresponding ones obtained via the popular hot-plate annealing. Accordingly, the fabricated devices display better performance than the corresponding ones fabricated via hot-plate annealing: higher PCE and higher uniformity. A high PCE of 14.9% is achieved (VOC of 1.02 V, JSC of 20.3 mA/cm2, FF of 0.72), which is amongst the highest for such device with the structure of Al/PC61BM/CH3NH3PbI3−xClx/PEDOT:PSS/ITO/glass. In contrast, the best device fabricated via hot-plate-annealing shows an inferior PCE of 13.5% (VOC of 0.98 V, JSC of 20.1 mA/cm2, FF of 0.69). We also systematically investigated the humidity effects on film morphology and device performance. The work demonstrates that air-heated-oven annealing we employed here could have great applications in future perovskite solar cell fabrications.
Experimental
CH3NH3I synthesis
Methylammonium iodide (MAI) was synthesized by reacting 10 mL hydroiodic acid (57% in water, Sigma-Aldrich), 24 mL methylamine (33% in absolute ethanol, Sigma-Aldrich), and 100 mL ethanol in a 250 mL round-bottomed flask at 0 °C for 2 h with stirring. The white precipitate of MAI was obtained by rotary evaporating the solution at 50 °C for about 1 h. The product was dissolved in ethanol, recrystallized by sedimentation in diethyl ether, and dried at 60 °C under vacuum for 24 h.
Precursor solution preparing
MAI and lead (II) chloride (PbCl2, Sigma-Aldrich, 99.9995%) were dissolved in anhydrous N,N-Dimethylformamide (DMF, Aldrich), with a molar ratio of 3:1 and a final weight percent of 30% in DMF. This solution was filtered through a 0.4-μm-pore PTFE filter after stirring at 60 °C for 24 h and stored under a dry nitrogen atmosphere.
Film depositing and annealing
The patterned ITO (15 ohm/sq) glass substrates were washed sequentially with detergent and deionized water, acetone, and isopropanol with ultrasonication for 10 min each, and then were dried and treated by O2 plasma. A dispersion of poly(3,4-ethylenedioxythiophene):poly(styrenesulfonic acid) (PEDOT:PSS, levios, Al 4083, filtered through a 0.45 μm nylon filter) was first spin-coated on the ITO substrate at 2000 rpm for 45 s and subsequently dried at 140 °C for 15 min in air. To avoid the effect of moisture on perovskite precursor solution during the fabrication of perovskite thin-film, the substrates were transferred into a N2 glove-box, where the perovskite film (about 260 nm thickness) was fabricated by spin-coating a 30 wt% CH3NH3PbI3−xClx precursor solution (PbCl2 + 3CH3NH3I) at 3000 rpm for 45 s. The samples were then transferred out of the N2 glove-box and put in a dry-air supplied glove-box, then were annealed in an air-heated oven (with forced air circulation, LABCAB, DHG9030A) (at 100 °C for 25 min) or on a hot plate (Stuart SD160, UK) (at 100 °C for 35 min) under various humidity condition (10% to 90% RH). The optimization of annealing process and characterizations of corresponding materials and films can be found in the supporting information Figure S1–S4.
Device fabrication
After annealing, the [6,6]-phenyl-C61-butyric acid methyl ester (PC61BM, 15 mg/mL in chlorobenzene) solution was spin-coated on top of the perovskite layer at 900 rpm for 18 s and 6000 rpm for 30 s sequentially in the N2 glove-box. Finally, 100 nm thick aluminum cathode electrode was deposited by thermal evaporation through a metal shadow mask, completing the fabrication of the device (Al/PC61BM/CH3NH3PbI3−xClx/PEDOT:PSS/ITO/ glass).
Relative Humidity Control
For all the humidity studies, the perovskite films were annealed in a 1.8 × 0.8 × 0.6 m3 controlled-humidity (measured at 26 ± 1 °C) air glove-box. The relative humidity was carefully adjusted and controlled by evaporating deionized water in dry-air supplied glove-box, and waiting for more than 30 min to reach equilibrium. The relative humidity was measured periodically using a hygrometer throughout the course of the experiments using a calibrated hygrometer (±2%).
Material and film Characterization
The X-ray diffraction (XRD) spectra of the PbI2 powder and the prepared films were measured using Rigaku-2500 X-ray diffractometer with an X-ray tube (Cu Kα, λ = 1.5406 Å). The morphology of the films was recorded using a scanning electron microscope (SEM, HITACH2100). X-ray photoelectron spectroscopy (XPS) was carried out in ESCALab250Xi XPS system by a MgKα X-ray source (1253.6 eV).
Device Characterization
External quantum efficiency (EQE) was recorded by a Newport Oriel IQE-200 by a power source (Newport 300 W Xenon lamp, 66920) with a monochromatic (Newport Cornerstone 260). All current density-voltage (J-V) curves were measured using a source meter (Keithley 2420, USA) under AM 1.5 sunlight at an irradiance of 100 mW/cm2 provided by a solar simulator (Newport, Oriel Sol3A Class AAA, 94043A). Light intensity was calibrated using a monocrystalline silicon reference cell with KG5 window (Newport, Oriel 91150). The J-V curves were measured by reverse (forward bias (1.2 V) → backward voltage (−0.8 V)) or forward (backward voltage (−0.8 V) → forward bias (1.2 V)) scan (with a rate of 200 mV/s). The device area was 0.168 cm2, determined by the overlap of the cathode and anode. In order to avoid the overestimation of the photocurrent density by the edge effect and the optical piping effect, the solar cells were masked with a metal aperture (area of 0.08 cm2) over the device (0.168 cm2) to define the active area38,39.
Device parameters statistics
Mean (average) values and standard deviations of VOC/JSC/FF/PCE for the CH3NH3PbI3−xClx perovskite solar cell based on both annealing approaches. Data were statistically analyzed with outlying observation using the descriptive statistics in Origin Version 9.0. Standard deviation is a widely used measure of variability and uniformity used in statistics theory. The smaller the standard deviation, the more uniform the parameter is.
Additional Information
How to cite this article: Zhou, Q. et al. Enhancing performance and uniformity of CH3NH3PbI3−xClx perovskite solar cells by air-heated-oven assisted annealing under various humidities. Sci. Rep. 6, 21257; doi: 10.1038/srep21257 (2016).
References
Kojima, A., Teshima, K., Shirai, Y. & Miyasaka, T. Organometal Halide Perovskites as Visible-Light Sensitizers for Photovoltaic Cells. J. Am. Chem. Soc. 131, 6050–6051 (2009).
Lee, M. M., Teuscher, J., Miyasaka, T., Murakami, T. N. & Snaith, H. J. Efficient Hybrid Solar Cells Based on Meso-Superstructured Organometal Halide Perovskites. Science 338, 643–647 (2012).
Burschka, J. et al. Sequential deposition as a route to high-performance perovskite-sensitized solar cells. Nature 499, 316–319 (2013).
Stranks, S. D. et al. Electron-Hole Diffusion Lengths Exceeding 1 Micrometer in an Organometal Trihalide Perovskite Absorber. Science 342, 341–344 (2013).
Jeon, N. J. et al. Solvent engineering for high-performance inorganic–organic hybrid perovskite solar cells. Nat. Mater. 13, 897–903 (2014).
Xiao, M. et al. A Fast Deposition-Crystallization Procedure for Highly Efficient Lead Iodide Perovskite Thin-Film Solar Cells. Angew. Chem. Int. Ed. 53, 9898–9903 (2014).
Yang, W. S. et al. High-performance photovoltaic perovskite layers fabricated through intramolecular exchange. Science 348, 1234–1247 (2015).
Wehrenfennig, C., Liu, M., Snaith, H. J., Johnston, M. B. & Herz, I. Charge-Carrier Dynamics in Vapour-Deposited Films of the Organolead Halide Perovskite CH3NH3PbI3−xClx . Energy Environ. Sci. 7, 2269–2275 (2014).
Liu, M., Johnston, M. B. & Snaith, H. J. Efficient planar heterojunction perovskite solar cells by vapour deposition. Nature 501, 395–398 (2013).
Chen, Q. et al. Planar Heterojunction Perovskite Solar Cells via Vapor-Assisted Solution Process. J. Am. Chem. Soc. 136, 622–625 (2013).
Zhang, W. et al. Ultrasmooth organic-inorganic perovskite thin-film formation and crystallization for efficient planar heterojunction solar cells. Nat Commun 6, 6142 (2015).
Docampo, P. et al. Solution Deposition-Conversion for Planar Heterojunction Mixed Halide Perovskite Solar Cells. Adv. Energy Mater. 4, 1400355 (2014).
Heo, J. H., Song, D. H. & Im, S. H. Planar CH3NH3PbBr3 Hybrid Solar Cells with 10.4% Power Conversion Efficiency, Fabricated by Controlled Crystallization in the Spin-Coating Process. Adv. Mater. 26, 8179–8183 (2014).
Barrows, A. T. et al. Efficient planar heterojunction mixed-halide perovskite solar cells deposited via spray-deposition. Energy Environ. Sci. 7, 2944–2950 (2014).
Wang, K., Liu, C., Du, P., Zhang, H. L. & Gong, X. Efficient Perovskite Hybrid Solar Cells Through a Homogeneous High-Quality Organolead Iodide Layer. Small 11, 3369–3376 (2015).
Chen, W. et al. Hybrid interfacial layer leads to solid performance improvement of inverted perovskite solar cells. Energy Environ. Sci. 8, 629–640 (2015).
Shen, D. et al. Understanding the solvent-assisted crystallization mechanism inherent in efficient organic-inorganic halide perovskite solar cells. J. Mater. Chem. A 2, 20454–20461 (2014).
Ku, Z., Rong, Y., Xu, M., Liu, T. & Han, H. Full Printable Processed Mesoscopic CH3NH3PbI3/TiO2 Heterojunction Solar Cells with Carbon Counter Electrode. Sci. Rep. 3, 3132 (2013).
Mei, A. et al. A hole-conductor–free, fully printable mesoscopic perovskite solar cell with high stability. Science 345, 295–298 (2014).
Hu, M. et al. Efficient hole-conductor-free, fully printable mesoscopic perovskite solar cells with a broad light harvester NH2CH=NH2PbI3 . J. Mater. Chem. A 2, 17115–17121 (2014).
Liu, L. et al. Fully Printable Mesoscopic Perovskite Solar Cells with Organic Silane Self-Assembled Monolayer. J. Am. Chem. Soc. 137, 1790–1793 (2015).
Chueh, C.-C., Li, C.-Z. & Jen, A. K. Y. Recent progress and perspective in solution-processed Interfacial materials for efficient and stable polymer and organometal perovskite solar cells. Energy Environ. Sci. 8, 1160–1189 (2015).
Xiao, Z. et al. Solvent Annealing of Perovskite-Induced Crystal Growth for Photovoltaic-Device Efficiency Enhancement. Adv. Mater. 26, 6503–6509 (2014).
Zhao, Y. & Zhu, K. Solution Chemistry Engineering toward High-Efficiency Perovskite Solar Cells. J. Phys. Chem. Lett. 5, 4175–4186 (2014).
Docampo, P., Ball, J. M., Darwich, M., Eperon, G. E. & Snaith, H. J. Efficient organometal trihalide perovskite planar-heterojunction solar cells on flexible polymer substrates. Nat. Commun. 4, 2761 (2013).
Leijtens, T. et al. Overcoming ultraviolet light instability of sensitized TiO2 with meso-superstructured organometal tri-halide perovskite solar cells. Nat. Commun. 4, 2885 (2013).
Smith, I. C., Hoke, E. T., Solis-Ibarra, D., McGehee, M. D. & Karunadasa, H. I. A Layered Hybrid Perovskite Solar-Cell Absorber with Enhanced Moisture Stability. Angew. Chem. 126, 11414–11417 (2014).
Kim, J. H., Williams, S. T., Cho, N., Chueh, C.-C. & Jen, A. K. Y. Enhanced Environmental Stability of Planar Heterojunction Perovskite Solar Cells Based on Blade-Coating. Adv. Energy Mater. 5, 1401229 (2015).
Seetharaman, S. M. et al. Efficient Organic Inorganic Hybrid Perovskite Solar Cells Processed in Air. Phys. Chem. Chem. Phys. 16, 24691–24696 (2014).
Lee, B. et al. Air-Stable Molecular Semiconducting Iodosalts for Solar Cell Applications: Cs2SnI6 as a Hole Conductor. J. Am. Chem. Soc. 136, 15379–15385 (2014).
You, J. et al. Moisture assisted perovskite film growth for high performance solar cells. Appl. Phys. Lett. 105, 183902 (2014).
Raga, S. R. et al. Influence of Air Annealing on High Efficiency Planar Structure Perovskite Solar Cells. Chem. Mater. 27, 1597–1603 (2015).
Christians, J. A., Miranda Herrera, P. A. & Kamat, P. V. Transformation of the Excited State and Photovoltaic Efficiency of CH3NH3PbI3 Perovskite upon Controlled Exposure to Humidified Air. J. Am. Chem. Soc. 137, 1530–1538 (2015).
Sheikh, A. D. et al. Atmospheric effects on the photovoltaic performance of hybrid perovskite solar cells. Sol. Energ. Mat. Sol. C. 137, 6–14 (2015).
Eperon, G. E., Burlakov, V. M., Docampo, P., Goriely, A. & Snaith, H. J. Morphological Control for High Performance, Solution-Processed Planar Heterojunction Perovskite Solar Cells. Adv. Funct. Mater. 24, 151–157 (2014).
Wang, B., Young Wong, K., Xiao, X. & Chen, T. Elucidating the Reaction Pathways in the Synthesis of Organolead Trihalide Perovskite for High-Performance Solar Cells. Sci. Rep. 5, 10557 (2015).
Christians, J. A., Manser, J. S. & Kamat, P. V. Best Practices in Perovskite Solar Cell Efficiency Measurements. Avoiding the Error of Making Bad Cells Look Good. J. Phys. Chem. Lett. 6, 852–857 (2015).
Ball, J. M. et al. Optical properties and limiting photocurrent of thin-film perovskite solar cells. Energy Environ. Sci. 8, 602–609 (2015).
Snaith, H. J. How should you measure your excitonic solar cells? Energy Environ. Sci. 5, 6513–6520 (2012).
Acknowledgements
The authors acknowledge the financial support by 973 Program (Grant No. 2014CB643600, 2014CB643503 and 2011CB932304), National Natural Science Foundation of China (61405208), the Strategic Priority Research Program of the Chinese Academy of Sciences (Grant No. XDB12030200) and the CAS/SAFEA International Partnership Program for Creative Research Teams.
Author information
Authors and Affiliations
Contributions
Q.Z. performed the experimental work, the data analysis and the experimental planning; The project was conceived, planned and supervised by Q.Z. and J.W.; Z.J. and H.L. performed SEM and XPS characterization. The manuscript was written and reviewed by Z.J. and J.W. All authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Zhou, Q., Jin, Z., Li, H. et al. Enhancing performance and uniformity of CH3NH3PbI3−xClx perovskite solar cells by air-heated-oven assisted annealing under various humidities. Sci Rep 6, 21257 (2016). https://doi.org/10.1038/srep21257
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep21257
This article is cited by
-
Lattice strain suppresses point defect formation in halide perovskites
Nano Research (2022)
-
Improved performance and reproducibility of perovskite solar cells by jointly tuning the hole transport layer and the perovskite layer deposition
Journal of Materials Science: Materials in Electronics (2018)
-
Effect of 6R and 12R lead iodide polytypes on MAPbI3 perovskite device performance
Journal of Materials Science: Materials in Electronics (2018)
-
Structural, optical and photoluminescence properties of hybrid metal–organic halide perovskite thin films prepared by a single step solution method
Journal of Materials Science: Materials in Electronics (2018)
-
Annealing Induced Re-crystallization in CH3NH3PbI3−xClx for High Performance Perovskite Solar Cells
Scientific Reports (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.