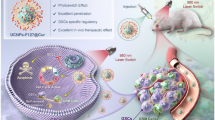
Abstract
The use of targeted nanoparticles (NPs) as a platform for loading photosensitizers enables selective accumulation of the photosensitizers in the tumor area, while maintaining their photodynamic therapy (PDT) effectiveness. Here two novel kinds of methylene blue (MB)-conjugated polyacrylamide (PAA) nanoparticles, MBI-PAA NPs and MBII-PAA NPs, based on two separate MB derivatives, are developed for PDT. This covalent conjugation with the NPs (i) improves the loading of MB, (ii) prevents any leaching of MB from the NPs and (iii) protects the MB from the effects of enzymes in the biological environment. The loading of MB into these two kinds of NPs was controlled by the input amount, resulting in concentrations with optimal singlet oxygen production. For each of the MB-NPs, the highest singlet oxygen production was found for an MB loading of around 11 nmol mg-1. After attachment of F3 peptide groups, for targeting, each of these NPs was taken up, selectively, by MDA-MB-435 tumor cells, in vitro. PDT tests demonstrated that both kinds of targeted NPs resulted in effective tumor cell kill, following illumination, while not causing dark toxicity.
Similar content being viewed by others
Notes and references
E. Buytaert, M. Dewaele and P. Agostinis, Molecular effectors of multiple cell death pathways initiated by photodynamic therapy, Biochim. Biophys. Acta, Rev. Cancer, 2007, 1776, 86–107.
M. M. Siegel, K. Tabei, R. S. Tsao, M. J. Pastel, R. K. Pandey, S. Berkenkamp, F. Hillenkamp and M. S. de Vries, Comparative mass spectrometric analyses of photofrin oligomers by fast atom bombardment mass spectrometry, UV and IR matrix-assisted laser desorption ionization mass spectrometry, electrospray ionization mass spectrometry and laser desorption/jet-cooling photoionization mass spectrometry, J. Mass Spectrom., 1999, 34, 661–669.
D. E. J. G. J. Dolmans, D. Fukumura and R. K. Jain, Photodynamic therapy for cancer, Nat. Rev. Cancer, 2003, 3, 380–387.
S. B. Brown, E. A. Brown and I. Walker, The present and future role of photodynamic therapy in cancer treatment, Lancet Oncol., 2004, 5, 497–508.
B. C. Wilson and M. S. Patterson, The physics, biophysics and technology of photodynamic therapy, Phys.Med. Biol., 2008, 53, R61–R109.
A. Juarranz, P. Jaen, F. Sanz-Rodriguez, J. Cuevas and S. Gonzalez, Photodynamic therapy of cancer. Basic principles and applications, Clin. Transl. Oncol., 2008, 10, 148–154.
D. Bechet, P. Couleaud, C. Frochot, M. L. Viriot, F. Guillemin and M. Barberi-Heyob, Nanoparticles as vehicles for delivery of photodynamic therapy agents, Trends Biotechnol., 2008, 26, 612–621.
G. S. Trindade, S. L. A. Farias, V. M. Rumjanek and M. A. M. Capella, Methylene blue reverts multidrug resistance: sensitivity of multidrug resistant cells to this dye and its photodynamic action, Cancer Lett., 2000, 151, 161–167.
K. J. Mellish, R. D. Cox, D. I. Vernon, J. Griffiths and S. B. Brown, In vitro photodynamic activity of a series of methylene blue analogues, Photochem. Photobiol., 2002, 75, 392–397.
D. Gabrielli, E. Belisle, D. Severino, A. J. Kowaltowski and M. S. Baptista, Binding, aggregation and photochemical properties of methylene blue in mitochondrial suspensions, Photochem. Photobiol., 2004, 79, 227–232.
R. D. Bongard, M. P. Merker, R. Shundo, Y. Okamoto, D. L. Roerig, J. H. Linehan and C. A. Dawson, Reduction of thiazine dyes by bovine pulmonary arterial endothelial-cells in culture, Am. J. Physiol., 1995, 13, L78–L84.
J. Umbreit, Methemoglobin—It’s not just blue: A concise review, Am. J. Hematol., 2007, 82, 134–144.
M. Wainwright, Non-porphyrin photosensitizers in biomedicine, Chem. Soc. Rev., 1996, 25, 351–359.
W. M. Sharman, C. M. Allen and J. E. van Lier, Photodynamic therapeutics: basic principles and clinical applications, Drug Discovery Today, 1999, 4, 507–517.
E. M. Tuite and J. M. Kelly, Photochemical Interactions of Methylene-Blue and Analogs with DNA and Other Biological Substrates, J. Photochem. Photobiol., B, 1993, 21, 103–124.
K. Orth, D. Russ, G. Beck, A. Ruck and H. G. Beger, Photochemotherapy of experimental colonic tumours with intra-tumorally applied methylene blue, Langenbecks Arch. Surg., 1998, 383, 276–281.
K. Orth, A. Rück, A. Stanescu and H. G. Beger, Intraluminal treatment of inoperable oesophageal tumours by intralesional photodynamic therapy with methylene blue, Lancet, 1995, 345, 519–520.
K. Orth, G. Beck, F. Genze and A. Ruck, Methylene blue mediated photodynamic therapy in experimental colorectal tumors in mice, J. Photochem. Photobiol., B, 2000, 57, 186–192.
J. M. May, Z. C. Qu and C. E. Cobb, Reduction and uptake of methylene blue by human erythrocytes, Am. J. Physiol.: Cell Physiol., 2004, 286, C1390–C1398.
K. Porkka, P. Laakkonen, J. A. Hoffman, M. Bernasconi and E. Ruoslahti, A fragment of the HMGN2 protein homes to the nuclei of tumor cells and tumor endothelial cells in vivo, Proc. Natl. Acad. Sci. U. S. A., 2002, 99, 7444–7449.
S. Christian, J. Pilch, M. E. Akerman, K. Porkka, P. Laakkonen and E. Ruoslahti, Nucleolin expressed at the cell surface is a marker of endothelial cells in angiogenic blood vessels, J. Cell Biol., 2003, 163, 871–878.
H. Maeda, The enhanced permeability and retention (EPR) effect in tumor vasculature: The key role of tumor-selective macromolecular drug targeting, Adv. Enzyme Regul., 2001, 41, 189–207.
W. Tang, H. Xu, R. Kopelman and M. A. Philbert, Photodynamic characterization and in vitro application of methylene blue-containing nanoparticle platforms, Photochem. Photobiol., 2005, 81, 242–249.
J. A. Harrel and R. Kopelman, Biocompatible probes measure intracellular activity, Biophotonics Int., 2000, 7, 22–24.
H. Xu, S. M. Buck, R. Kopelman, M. A. Philbert, M. Brasuel, B. D. Ross and A. Rehemtulla, Photoexcitation-based nano-explorers: Chemical analysis inside live cells and photodynamic therapy, Isr. J. Chem., 2004, 44, 317–337.
B. Ross, A. Rehemtulla, Y.-E. L. Koo, R. Reddy, G. Kim, C. Behrend, S. Buck, R. J. Schneider, M. A. Philbert, R. Weissleder and R. Kopelman, Photonic and magnetic nanoexplorers for biomedical use: fromsubcellular imaging to cancer diagnostics and therapy, Proc.SPIE, 2004, 5331, 76–83.
R. Kopelman, Y.-E. L. Koo, M. Philbert, B. A. Moffat, G. R. Reddy, P. McConville, D. E. Hall, T. L. Chenevert, M. S. Bhojani, S. M. Buck, A. Rehemtulla and B. D. Ross, Multifunctional nanoparticle platforms for in vivo MRI enhancement and photodynamic therapy of a rat brain cancer, J. Magn. Magn. Mater., 2005, 293, 404–410.
G. R. Reddy, M. S. Bhojani, P. McConville, J. Moody, B. A. Moffat, D. E. Hall, G. Kim, Y.-E. L. Koo, M. J. Woolliscroft, J. V. Sugai, T. D. Johnson, M. A. Philbert, R. Kopelman, A. Rehemtulla and B. D. Ross, Vascular targeted nanoparticles for imaging and treatment of brain tumors, Clin. Cancer Res., 2006, 12, 6677–6686.
Y.-E. L. Koo, G. R. Reddy, M. Bhojani, R. Schneider, M. A. Philbert, A. Rehemtulla, B. D. Ross and R. Kopelman, Brain cancer diagnosis and therapy with nanoplatforms, Adv. Drug Delivery Rev., 2006, 58, 1556–1577.
D. Peer, J. M. Karp, S. Hong, O. C. Farokhzad, R. Margalit and R. Langer, Nanocarriers as an emerging platform for cancer therapy, Nat. Nanotechnol., 2007, 2, 751–760.
M. E. Davis, Z. Chen and D. M. Shin, Nanoparticle therapeutics: an emerging treatment modality for cancer, Nat. Rev. Drug Discovery, 2008, 7, 771–782.
W. Tang, H. Xu, E. J. Park, M. A. Philbert and R. Kopelman, Encapsulation of methylene blue in polyacrylamide nanoparticle platforms protects its photodynamic effectiveness, Biochem. Biophys. Res. Commun., 2008, 369, 579–583.
M. Susa, A. K. Iyer, K. Ryu, F. J. Hornicek, H. Mankin, M. M. Amiji and Z. F. Duan, Doxorubicinloaded Polymeric Nanoparticulate Delivery System to overcome drug resistance in osteosarcoma, BMC Cancer, 2009, 9, 399.
M. D. Chavanpatil, A. Khdair, Y. Patil, H. Handa, G. Z. Mao and J. Panyam, Polymer-surfactant nanoparticles for sustained release of water-soluble drugs, J. Pharm. Sci., 2007, 96, 3379–3389.
A. Khdair, B. Gerard, H. Handa, G. Mao, M. P. V. Shekhar and J. Panyam, Surfactant-polymer nanoparticles enhance the effectiveness of anticancer photodynamic therapy, Mol. Pharmaceutics, 2008, 5, 795–807.
A. Khdair, H. Handa, G. Z. Mao and J. Panyam, Nanoparticlemediated combination chemotherapy and photodynamic therapy overcomes tumor drug resistance in vitro, Eur. J. Pharm. Biopharm., 2009, 71, 214–222.
H. J. Hah, G. Kim, Y.-E. Koo Lee, D. A. Orringer, O. Sagher, M. A. Philbert and R. Kopelman, Methylene blue-conjugated hydrogel nanoparticles and tumor-cell targeted photodynamic therapy, Macromol. Biosci., 2011, 11, 90–99.
M. J. Moreno, E. Monson, R. G. Reddy, A. Rehemtulla, B. D. Ross, M. Philbert, R. J. Schneider and R. Kopelman, Production of singlet oxygen by Ru(dpp(SO3)2)3 incorporated in polyacrylamide PEBBLES, Sens. Actuators, B, 2003, 90, 82–89.
I. Winer, S. Wang, Y.-E. Koo Lee, W. Fan, Y. Gong, D. Burgos-Ojeda, G. Spahlinger, R. Kopelman and R. J. Buckanovich, F3-targeted cisplatinhydrogel nanoparticles as an effective therapeutic that targets both murine and human ovarian tumor endothelial cells in vivo, Cancer Res., 2010, 70, 8674–8683.
M. K. Carroll, M. A. Unger, A. M. Leach, M. J. Morris, C. M. Ingersoll and F. V. Bright, Interactions between methylene blue and sodium dodecyl sulfate in aqueous solution studied by molecular spectroscopy, Appl. Spectrosc., 1999, 53, 780–784.
K. Patil, R. Pawar and P. Talap, Self-aggregation ofMethylene Blue in aqueous medium and aqueous solutions of Bu4NBr and urea, Phys. Chem. Chem. Phys., 2000, 2, 4313–4317.
A. Ghanadzadeh, A. Zeini, A. Kashef and M. Moghadam, Concentration effect on the absorption spectra of oxazine1 and methylene blue in aqueous and alcoholic solutions, J. Mol. Liq., 2008, 138, 100–106.
D. A. Orringer, Y.-E. L. Koo, T. Chen, G. Kim, H. J. Hah, H. Xu, S. Y. Wang, R. Keep, M. A. Philbert, R. Kopelman and O. Sagher, In vitro characterization of a targeted, dye-loaded nanodevice for intraoperative tumor delineation, Neurosurgery, 2009, 64, 965–971.
Y.-E. Koo Lee, E. E. Ulbrich, G. Kim, H. Hah, C. Strollo, W. Z. Fan, R. Gurjar, S. M. Koo and R. Kopelman, Near infrared luminescent oxygen nanosensors with nanoparticlematrix tailored sensitivity, Anal. Chem., 2010, 82, 8446–8455.
L. E. van Vlerken, T. K. Vyas and M. M. Amiji, Poly(ethylene glycol)-modified nanocarriers for tumor-targeted and intracellular delivery, Pharm. Res., 2007, 24, 1405–1414.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is published as part of a themed issue on immunological aspects and drug delivery technologies in PDT.
Rights and permissions
About this article
Cite this article
Qin, M., Hah, H.J., Kim, G. et al. Methylene blue covalently loaded polyacrylamide nanoparticles for enhanced tumor-targeted photodynamic therapy. Photochem Photobiol Sci 10, 832–841 (2011). https://doi.org/10.1039/c1pp05022b
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1039/c1pp05022b