Abstract
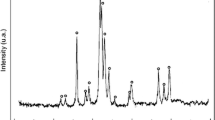
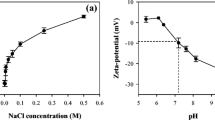
Milk and dairy products contain large amounts of calcium phosphate salts that can precipitate. The chemical composition and the crystalline structure of the calcium phosphate precipitates that are formed in dairy industry depend on the physico-chemical conditions, particularly, pH and mineral composition. The objective of this study was to determine, using mineral solutions, the effects of pH and of the concentrations of calcium and phosphate on the quantity and crystalline structure of calcium phosphate precipitates. Experiments were carried out at 20 °C with 20.00 mmol·L−1 phosphate and three Ca/P molar ratios (1.00, 1.50 and 2.00). The initial pH (5.50, 6.70, 7.50, 8.50 and 9.50) were drifting or kept constant during a reaction time of 3 h. After filtration of the suspensions, the mineral compositions of filtrates were quantified. The lyophilized precipitates were characterized using X-ray diffraction, infrared spectroscopy and scanning electron microscopy techniques. At drifting pH (final pH values were between 4.6 and 6.0), the mineral analyses showed that the Ca/P ratio did not influence the amounts of precipitated calcium and phosphate. The analyses of precipitates revealed the formation of brushite as the main crystalline phase. At constant pH, the mineral analyses showed that the Ca/P ratio strongly influenced the precipitation efficiency of calcium phosphate. The analyses of precipitates revealed the formation of poorly crystallized calcium-deficient apatites. A decrease of crystallinity with an increase in initial pH was observed. In conclusion, pH can be a key factor to control the quantity and crystalline structure of calcium phosphates obtained by precipitation. This factor should be considered for the recovery of calcium phosphates from dairy co-products. pH is also important in the fouling phenomena of membranes and heat exchangers caused by calcium phosphate precipitation.
Abstract
pH pH 20 °C 20.00 mmol·L−1 3 Ca/P (1.00 1.50 2.00) pH (5.50 6.70 7.50 8.50 9.50) 3 X- pH pH 4.6 6.0) Ca/P (CaHPO4·2H2O2) pH Ca/P pH pH pH
Résumé
Le lait et les produits laitiers sont riches en sels de phosphate de calcium qui peuvent précipiter. La composition chimique et la structure cristalline de ces précipités formés en industrie laitière dépendent des conditions physicochimiques de formation, notamment le pH et la composition minérale. L’objectif de ce travail était de déterminer, en utilisant des solutions minérales, les rôles du pH et des concentrations du calcium et du phosphate sur la nature et la quantité des précipités de phosphate de calcium. Les expériences ont été réalisées à 20 °C, avec une concentration en phosphate de 20.00 mmol·L−1 et trois rapports molaires Ca/P (1,00, 1,50 et 2,00). Les pH initiaux (5,50, 6,70, 7,50, 8,50 et 9,50) étaient dérivants ou maintenus constants pendant un temps de réaction de 3 heures. Après filtration des suspensions, les compositions minérales des filtrats ont été analysées. Les précipités lyophilisés ont été caractérisés par diffraction des rayons X, spectroscopie infrarouge et microscopie électronique à balayage. À pH dérivant (pH final des suspensions compris entre 4,6 et 6,0), l’analyse minérale a montré que le rapport Ca/P n’influençait pas les quantités de phosphate et de calcium précipitées. Les analyses des précipités ont montré la formation de brushite comme principale phase cristalline. À pH constant, l’analyse minérale a montré que le rapport Ca/P avait une forte influence sur le taux de précipitation du phosphate de calcium. Les analyses des précipités ont mis en évidence la formation d’apatites déficientes en calcium mal cristallisées. Une diminution de la cristallinité avec l’augmentation du pH initial a été observée. En conclusion, le pH peut être considéré comme un facteur essentiel pour contrôler la quantité et la structure cristalline des phosphates de calcium obtenus par précipitation. Ce facteur est à considérer dans la récupération des phosphates de calcium des co-produits laitiers. Le pH est également important dans les phénomènes d’encrassement des membranes et des échangeurs thermiques suite à la précipitation du phosphate de calcium.
Similar content being viewed by others
References
Andritsos N., Yiantsios S.G., Karabelas A.J., Calcium phosphate scale formation from simulated milk ultrafiltrate solutions, Food Bioprod. Process. 80 (2002) 223–230.
Calco M., Valorization of industrial wheys using membrane processes, Ph.D. Thesis, Université de Nancy 1, Nancy, France, 1997.
Elliott J.C., Structure and chemistry of the apatite and other calcium orthophosphates, Elsevier Science B.V., Amsterdam, 1994.
Famery R., Richard N., Boch P., Preparation of α- and β-tricalcium phosphate ceramics, with and without magnesium addition, Ceram. Int. 20 (1994) 327–336.
Ferreira A., Oliveira C., Rocha F., The different phases in the precipitation of dicalcium phosphate dehydrate, J. Cryst. Growth 252 (2003) 599–611.
Gaucheron F., Le Gräet Y., Piot M., Boyaval E., Determination of anions of milk by ion chromatography, Lait 76 (1996) 433–443.
Gesan G., Daufin G., Merin U., Labbé J.P., Quémerais A., Fouling during constant flux crossflow microfiltration of pretreated whey, Influence of transmembrane pressure gradient, J. Membr. Sci. 80 (1993) 131–145.
Giulietti M., Seckler M.M., Derenzo S., Re M.I., Cekinski E., Industrial crystallization and precipitation from solutions: state of the technique, Braz. J. Chem. Eng. 18 (2001) 423–440.
Guo L.F., Wang W.H., Zjang W.G., Wang C.T., Effects of synthesis factors on the morphology, crystallinity and crystal size of hydroxyapatite precipitation, J. Harbin Inst. Technol. 12 (2005) 656–660.
Harding I.S., Rashid N., Hing K.A., Surface charge and the effect of excess calcium ions on the hydroxyapatite surface, Biomaterials 26 (2005) 6818–6826.
Johnsson M.S.A., Nancollas G.H., The role of brushite and octacalcium phosphate in apatite formation, Crit. Rev. Oral Biol. Med. 3 (1992) 61–82.
Joko I., Phosphorus removal from wastewater by the crystallization method, Water Sci. Technol. 17 (1984) 121–132.
Labbe J.P., Quemerais A., Michel F., Daufin G., Fouling of inorganic membranes during whey ultrafiltration: analytical methodology, J. Membr. Sci. 165 (1990) 293–307.
LeGeros R.Z., Calcium phosphates in oral biology and medicine, New York University College of Dentistry, New York, 1991.
Madsen H.E.L., Christensson F., Precipitation of calcium phosphate at 40 °C from neutral solution, J. Cryst. Growth 114 (1991) 613–618.
Madsen H.E.L., Thorvardarson G., Precipitation of calcium phosphate from moderately acid solution, J. Cryst. Growth 66 (1984) 369–376.
Marshall R.W., Nancollas G.H., The kinetics of crystal growth of dicalcium phosphate dehydrate, J. Phys. Chem. 73 (1969) 3838–3844.
Mc Dowell H., Gregory T.M., Brown W.E., Solubility of Ca5(PO4)3OH in the system Ca(OH)2-H3PO4-H2O at 5, 15, 25, and 37 °C, J. Res. Nat. Bur. Stand. 81 (1977) 273–281.
Nancollas G.H., Physical chemistry of crystal nucleation, growth and dissolution of stones, in: Wickham J.E.A., Buck A.C. (Eds.), Renal Tract Stone, Metabolic Basis and Clinical Practice, Churchill Livingstone, Edinburgh, 1990, pp. 71–85.
Nancollas G.H., Lore M., Perez L., Richardson C., Zawacki S.J., Mineral phases of calcium phosphate, Anat. Rec. 224 (1989) 234–241.
Orme C., Giocondi J., Model systems for formation and dissolution of calcium phosphate minerals, in: Behrens P., Baeuerlein E. (Eds.), Handbook of Biomineralization: Biomimetic and Bio-inspired Materials Chemistry, Wiley-VCH, Weinheim, 2007, pp. 135–157.
Pouliot Y., Landry J., Giasson J., Induction of calcium phosphate precipitation in sweet whey permeate, Lait 71 (1991) 313–320.
Raynaud S., Champion E., Bernache-Assollant D., Thomas P., Calcium phosphate apatites with variable Ca/P atomic ratio. I. Synthesis, characterisation and thermal stability of powders, Biomaterials 23 (2002) 1065–1072.
Rice G., Kentish S., O’Connor A., Stevens G., Lawrence N., Barber A., Fouling behaviour during the nanofiltration of dairy ultrafiltration permeate, Desalination 199 (2006) 239–241.
Rosmaninho R., Rizzo G., Muller-Steinhagen H., Melo L.F., Deposition from a milk mineral solution on novel heat transfer surfaces under turbulent flow conditions, J. Food Eng. 85 (2008) 29–41.
Saulnier F., Ferrero F., Bottero J.Y., Linden G., Variation of the composition and nature of the insoluble precipitate from industrial wheys, Lait 75 (1995) 93–100.
Schmidt D.G., Both P., Studies on the precipitation of calcium phosphate. I. Experiments in the pH range 5.3 to 6.8 at 25 °C and 50 °C in the absence of additives, Neth. Milk Dairy J. 41 (1987) 105–119.
Smith R.M., Mattell A.E., Critical stability constants, Vols. 1–6, Plenum Press, New York, 1976.
Spanos N., Patis A., Kanellopoulou D., Andritsos N., Koutsoukos P.G., Precipitation of calcium phosphate from simulated milk ultrafiltrate solutions, Cryst. Growth Des. 7 (2007) 25–29.
Suvorova E.I., Buffat P.A., Electron diffraction and high resolution transmission electron microscopy in the characterization of calcium phosphate precipitation from aqueous solutions under biomineralization conditions, Eur. Cell. Mater. 1 (2001) 27–42.
Tung M.S., Calcium phosphates: structure, composition, solubility, and stability, in: Amjad Z. (Ed.), Calcium phosphates in biological and industrial systems, Kluwer Academic Publishers, Norwell, Massachusetts, 1998, pp. 1–19.
Valsami-Jones E., Mineralogical controls on phosphorus recovery from wastewaters, Mineral. Mag. 65 (2001) 611–620.
Van der Houwen J.A.M., Cressey G., Cressey B.A., Valsami-Jones E., The effect of organic ligands on the crystallinity of calcium phosphate, J. Cryst. Growth 249 (2003) 572–583.
Van der Houwen J.A.M., Valsami-Jones E., The application of calcium phosphate precipitation chemistry to phosphorus recovery: the influence of organic ligands, Environ. Technol. 22 (2001) 1325–1335.
Van Kemenade M.J.J.M., De Bruyn P.L., A kinetic study of precipitation from supersaturated calcium-phosphate solutions, J. Col. Interface Sci. 118 (1987) 564–585.
Vereecke G., Lemaitre J., Calculation of the solubility diagrams in the system Ca(OH)2-H3PO4-KOH-HNO3-CO2-H2O, J. Cryst. Growth 104 (1990) 820–832.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Mekmene, O., Quillard, S., Rouillon, T. et al. Effects of pH and Ca/P molar ratio on the quantity and crystalline structure of calcium phosphates obtained from aqueous solutions. Dairy Sci. Technol. 89, 301–316 (2009). https://doi.org/10.1051/dst/2009019
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1051/dst/2009019