Abstract
The relationship between the morphology of carbon-based nanomaterials (CBNs) and the specific response of plants exposed to CBNs has not been studied systematically. Here, we prove that CBNs with different morphologies can activate cell growth, germination, and plant growth. A tobacco cell culture growth was found to increase by 22%–46% when CBNs such as helical multi-wall carbon nanotubes (MWCNTs), few-layered graphene, long MWCNTs, and short MWCNTs were added to the growth medium at a concentration of 50 μg ml−1. The germination of exposed tomato seeds, as well as the growth of exposed tomato seedlings, were significantly enhanced by the addition of all tested CBNs. The presence of CBNs inside exposed seeds was confirmed by transmission electron microscopy and Raman spectroscopy. The effects of helical MWCNTs on gene expression in tomato seeds and seedlings were investigated by microarray technology and real time-PCR. Helical MWCNTs affected a number of genes involved in cellular and metabolic processes and response to stress factors. It was shown that the expression of the tomato water channel gene in tomato seeds exposed to helical MWCNTs was upregulated. These established findings demonstrate that CBNs with different morphologies can cause the same biological effects and share similar mechanisms in planta.
Export citation and abstract BibTeX RIS
1. Introduction
The research area of plant–nanoparticle interactions has attracted a lot of interest in recent years. While certain studies have validated the use of nanotechnology in horticulture and agriculture [1–6], others have raised questions concerning the potential adverse effects of nanoparticles (NPs) on humans and environmental health [7–10]. Initial reports presented the unique properties of nanomaterials and enumerated their successful uses as pesticides [11], seed/plant growth enhancers [12, 13], and carriers of herbicides, fertilizers, DNA, and phytohormones to plant cells [3, 14, 15]. Although research in this interdisciplinary field is promising, it remains imperative to understand the effect of nanoparticle properties on the particular plant response.
Many studies have shown that the bioresponse of different organisms to the application of nanomaterials is dependent on the specific properties of the added NPs [16–20]. The positive or negative effects observed in organisms exposed to nanomaterials were ultimately related to the size [19, 21, 22], shape [21], concentration [5], and surface chemistry [21, 23] of the NPs. For instance, the maximum uptake of nanomaterials by mammalian cells was recorded when spherical gold NPs, silica NPs, single-walled carbon nanotubes, or quantum dots had a 50 nm diameter [16, 19]. On the other hand, the ligand or the molecules used to coat the NPs play a vital role in the observed biological responses. In fact, functional groups on the nanoparticle have to initially interact with the negatively charged heparan sulfate proteoglycan groups on the exterior of the cells and subsequently enter the cell. Thus, the different physiochemical properties can either enhance or delay the spontaneous endocytosis of these materials [24]. The overall scope of work using mammalian cells has shown that small, elongated, and positively charged NPs can penetrate preferentially to big, flat, and uncharged NPs [24]. Very little is known about the effects of nanomaterials on plants or plant cells, partly due to the presence of cell walls in plant cells.
The interaction of plant cells with NPs has been discussed in several reviews [25–27]. Most of the studies clearly indicate the impact of the chemical structure, size, shape, concentration, and surface area of the NPs on uptake and plant response. For example, soybean treated with ZnO (8 nm) at 500 μg ml−1 had increased root growth and the treatment was not toxic to plant growth [28]; however, the same size ZnO particles at 2000–4000 μg ml−1 were found to decrease root growth [28]. Moreover, it was shown that more negatively charged multi-wall carbon nanotubes (MWCNTs) were more efficient in increasing seed germination and fresh weight than less negatively charged MWCNTs [23]. Different size ceria NPs were shown to have different biodistribution within cucumber plants. While 7 nm spherical nanomaterials were able to enter the root system and translocate within the plant, 25 nm ceria NPs were only detected on the outer surface of the cucumber roots [29]. Other reports have shown that gold NPs with a diameter of 3.5 nm entered tobacco root systems while larger 18 nm particles failed to get into the root epidermis [30]. The discussed reports are associated with metal-based nanoparticles (MBNs). However, there are no clear data on whether the size/shape of carbon-based nanomaterials (CBNs) can play a role in the response of plants to the application of nanomaterials.
While current reports have tested many different CBNs on plant seeds or seedlings, there is no indication of whether the morphology of these materials is among the contributors to the effects observed. A significant increase of germination, fresh biomass, and the length of the stem was noted when tomato seeds were exposed to a medium containing a low concentration of MWCNTs [23, 31]. Higher levels of MWCNTs (1000–2000 mg l−1) were toxic and resulted in a reduction of biomass (38%) in zucchini and root length in lettuce [32, 33]. On the other hand, plant responses on the application of CBNs were studied using fullerol [20, 34–36], functionalized and non-functionalized MWCNTs [12, 13, 23, 31], single-walled carbon nanotubes (SWCNTs) [4, 8], graphene [37], and single-walled carbon nanohorns (SWCNHs) [2]. However, the indicated experiments were performed in different experimental settings and using different plant models. Therefore, a carefully studied comparative investigation of the effect of CBNs with different morphologies on the same biosystem is required.
The major goal of this work is to understand whether the morphology of CBNs can play a key role in the response of plants exposed to nanomaterials. Our aim was to investigate the effect of different types of commercially available carbon-based nanomaterials on seed germination, plant development, and the cell growth process. To achieve this goal, we tested the physiological and genetic response of a dicot model plant (tomato, cv. Micro-Tom) and added four different types of CBN: few-layered graphene, helical MWCNTs, long MWCNTs, and short MWCNTs.
2. Materials and methods
2.1. Materials
Graphene nanoplatelets (<3 layers; lateral dimensions 1–2 μm), long MWCNTs (MWCNT-COOH-long (OD 13–18 nm; length 1–12 μm)), and short MWCNTs (MWCNT-COOH-short (OD < 7 nm; length 0.5–2 μm)) were purchased from Cheap Tubes (Brattleboro, VT). Helical carbon nanotubes (Helical MWCNTs (OD 100–200 nm; length 1–10 μm)) were obtained from US Research Nanomaterials Inc. (Houston, TX). Lycopersicon esculentum seeds (cv. Micro-Tom) were purchased from Reimer Seeds Inc. (Saint Leonard, MD).
2.2. Exposure of callus to CBNs
The tobacco callus cell culture was established as previously described [2]. The tobacco callus was exposed to growth medium supplemented with CBNs in vitro glass test tubes (Phytotechnology Laboratories, Inc.). Murashige and Skoog (MS) medium with 1 mg l−1 2,4-D was used as the control. The tobacco cell culture was exposed to a second control medium composed of the MS medium supplemented with activated carbon (AC) at concentrations of 50 and 100 μg ml−1. The experimental CBN medium was prepared by addition helical MWCNTs, graphene, long MWCNTs, or short MWCNTs at concentrations of 50 and 100 μg ml−1 with MS plus 2,4-D base. A similar callus inoculum amount (300 mg) was added on top of the medium containing agar, and experimental tubes were incubated in the dark for 30 days. Different biological replicates were used for a total of ten technical replicates (n = 10).
2.3. Seed germination and seedling growth
Germination and growth experiments were performed under sterile conditions. Tomato seeds were washed with 80% EtOH and further kept in 50% bleach for 15 min. Seeds were finally washed thoroughly in deionized autoclaved water and placed in Magenta boxes containing growth medium. The experimental medium was composed of MS medium supplemented with CBNs at concentrations of 50 and 100 μg ml−1. The controls used included MS medium without CBNs and MS medium supplemented with AC at concentrations of 50 and 100 μg ml−1. Seed germination was monitored for ten consecutive days and the percentage of seed germinated was calculated. At day 20, seedlings were collected for phenotypic analysis. The different parameters measured included root/shoot lengths and fresh/dry weights of shoots and roots. Each experiment condition was repeated three times (five technical replicates at each condition).
2.4. Statistical analysis of seed germination and plant phenotype
The data associated with germination and plant growth are represented by the mean values calculated. Error bars represent standard error values. The difference between two treatments is considered significant if it meets the statistical significance of p < 0.05. SPSS® software was used for performing ANOVA and post hoc analysis as well as repeated measures using ANOVA.
2.5. Detection of CBNs in seed endosperm by Raman spectroscopy
A Renishaw 1000 confocal Raman spectrometer was used to measure the response of 1D and 2D carbon structures. The system was calibrated using a silicon substrate as a reference. Raman scattering was collected in backscattering configuration using a 633 nm laser for excitation.
2.6. Detection of long MWCNTs inside tomato seeds using TEM
After 24 h incubation, seeds exposed to long MWCNTs, as well as seeds exposed to the control medium, were dissected, and the endosperm was isolated. Grids were prepared as previously described [2]. The sections were examined by transmission electron microscope (JEOL 2100 FE).
2.7. Microarray analysis of the gene expression in tomato seeds and seedlings exposed to helical MWCNTs
The gene expression of seeds and seedlings exposed to helical MWCNTs was monitored using the Affymetrix tomato genome arrays methodology (www.affymetrix.com). Two different plant tissues were selected for microarray analysis including tomato seeds (exposed for 24 h to helical MWCNTs) and seedlings (exposed for 11 days to helical MWCNTs). The corresponding controls of seeds and seedlings exposed to the control medium were used in the microarray analysis. Plant tissues (seeds and seedlings) were snap frozen and powdered using a mortar and pestle. Total RNA was isolated using a RNeasy Plant Mini Kit (Qiagen Inc., Valencia, CA) and then treated with DNase to remove DNA residuals using the Qiagen RNase-free DNase Kit. The total transcriptome analysis was performed as previously described by Lahiani et al [2]. Briefly, biotinylated cRNA targets were synthesized following Affymetrix recommendations (Affymetrix IVT Express target labeling assay) and hybridization was performed by Expression Analysis, Inc. Three genes were selected for the confirmation of microarray data including (a) Les.50.1.S1_at ribonuclease, (b) Les.3635.1S1_at Subtilise-like protease, and (c) Les.1841.1.S1_at 1-aminocyclopropane-1-carboxylate synthase. Synthesis of cDNA for all RNA samples was carried out using a SuperScript III First-Strand Synthesis System Kit (Invitrogen, Carlsbad, CA) with dT16-oligonucleotide primers according to the manufacturer's protocol. The expression of all three genes was validated by RT-PCR as previously described by Khodakovskaya et al [31]. The primers designed for the amplification of all three genes were designed using IVT online software as described previously by Lahiani et al [2]. The qPCR reaction was run for 55 cycles and followed by a complete denaturation of the PCR product to obtain melting curves. Melting curve data were obtained from 60 to 95 °C at a rate of 0.5 °C s−1 and allowed the confirmation of specificity of the amplicon product. All data were normalized to the internal standard control 18S. The corresponding primers of the 18S gene were 5'-AGGCCGCGGAAGTTTGAGGC-3' (forward primer) and 5'-ATCAGTGTAGCGCGCGTGGG-3' (reverse primer). Three biological replicates were used for the analysis with three technical replicates in each condition.
2.8. The statistical analysis of gene expression data and associated biological processes
The microarray data were analyzed using TM4® software. Data were normalized prior to analysis using a generalized logarithm transformation (log2) and using the control group as a reference. The differences of expression were considered significant if it passed the unpaired t-test analysis with a p-value of 0.01. All genes with known functions were represented by heat map. A color gradient was used to show the level by which a gene was down- or upregulated compared to the control sample. To identify the biological processes altered by the exposure of seeds or seedlings to helical MWCNTs, all significantly altered genes were analyzed using the Tomato Functional Genomic Database (http://ted.bti.cornell.edu/cgiin/TFGD/array/funcat.cgi). The generated microarray data were placed in the public database GEO under accession number GSE70842.
2.9. RT-PCR analysis of aquaporin expression seeds exposed to helical MWCNTs
The expression of aquaporin in seeds exposed to helical MWCNTs was monitored in a real-time manner. Tomato seeds were exposed to helical MWCNTs at 50 μg ml−1 and samples were collected at 12, 24, and 48 h post-exposure. Control seeds exposed to control media were collected in a similar fashion. RNA was extracted, and cDNA was synthesized as described in section 2.7. Following synthesis, cDNA was used for the PCR reaction using gene-specific primers. The primers for the tomato aquaporin gene used are F-5'TACTGCACTGCTGGCATATCAGGT3' and R-5'AGAACACTGCCCTGGTTAAGGACA3'. The internal control used is 18S. Amplification of genes was carried out for 30 cycles and the PCR products were separated by electrophoresis for 30 min at 5 V cm–1.
3. Results and discussion
3.1. Characterization of carbon-based nanoparticles
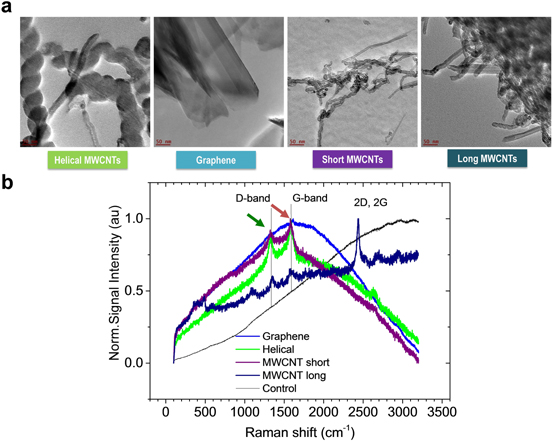
The CBNs were characterized using various microscopy and spectroscopy techniques. Figure 1(a) shows high-resolution TEM images of the helical MWCNTs, graphene sheets, short MWCNTs, and long MWCNTs. The helical MWCNTs had an outer diameter of 100–200 nm and a length of 1–10 μm; graphene nanoplatelets were less than three layers and had lateral dimensions of 1–2 μm; long MWCNTs were functionalized with a carboxylic group, had an outer diameter of 13–18 nm and were 1–12 μm long; and short carbon nanotubes were functionalized with a carboxylic group and had a 20–30 nm outer diameter size with a length of 0.5–2 μm. The purity of all materials was checked using the thermogravimetric analysis (TGA) technique. Figure S1 in the supplemental section shows the TGA curves of different CBNs. The purity of helical MWCNTs, graphene, short MWCNTs, and long MWCNTs was found to be 97%, 95%, 80%, and 95%, respectively. The first derivative (DTA) of the TGA curve is indicative of the presence of amorphous and crystalline carbonaceous materials. The decomposition temperatures of the different nanomaterials determined by the TGA curve were: 528 °C (helical MWCNTs), 607 °C (graphene), 550 °C (short MWCNTs), and 735 °C (long MWCNTs). The helical MWCNTs decomposed at the lowest temperature compared to the other nanostructures, indicating the presence of graphitic materials with reduced crystallinity. The low-temperature decomposition of the short MWCNTs could be a result of three main contributing factors: (1) their smaller size and diameter (shorter nanotubes with fewer walls decompose at a faster rate); (2) the presence of non-crystalline features on the side walls (during the functionalization process the C–C bonds are broken); and (3) the presence of impurities (the 80% purity analysis indicates the presence of 20% impurities, which might be the catalyst the nanoparticles used to synthesize the nanotubes [38]). All tested nanomaterials were also analyzed by Raman spectroscopy once they were submerged in media. The medium containing CBNs was air dried on glass slides and then visualized and analyzed using Raman spectroscopy (figure 1(b)). To probe the suitability of the laser excitation we sampled CBNs dispersed in growth media. Figure 1(b) shows a series of Raman spectra of graphene, short, long, and helical MWCNTs. The Raman characteristic features of nanotubes and graphene, the G-band (tangential mode present in carbon materials) and D-band (disorder-induced mode), overlap with the broad feature (peaking around 1500 cm−1) that might arise from the growth media. The D-band is a wavelength-dependent mode, which occurs due to defects and the presence of non-crystalline carbon in the graphitic structures [39]. The relatively high-intensity D-band present in the Raman spectra of the short MWCNTs corresponds to the defects present on the walls of the nanotubes as a result of the post-treatment and the addition of functional groups. This nicely correlates with the TGA/DTA of the short MWCNTs, which indicated a low decomposition temperature and the presence of other carbonaceous products in the nanotube sample.
Figure 1. Characterization of CBNs used for biological experiments. (a) High-resolution TEM images of helical MWCNTs, graphene, short MWCNTs, and long MWCNTs. (b) Confocal Raman spectra of short, long, and helical MWCNTs and graphene in growth media measured with 633 nm excitation. Characteristic features of sp2 carbon 1325 (green arrow) and 1580 cm-1 (red arrow) of D- and G-bands in all CBNs.
Download figure:
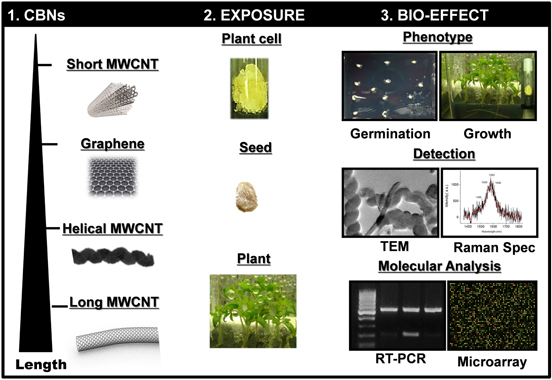
Standard image High-resolution imageTo understand whether the morphology of carbonaceous nanomaterials could play a significant role in the type of biological response of the exposed plant tissues, we designed a complex experiment for the comparative investigation of the effect of different CBNs at the cellular level (tobacco callus culture) and the organism level (tomato seeds, young plants) (figure 2). The experiment design contains three major steps: (1) the preparation of the medium mixed with different types of CBNs at various concentrations; (2) the exposure of tobacco cell culture, tomato seeds, and seedlings to growth media containing different CBNs; and (3) the analysis of the bioeffects resulting from the exposure of CBNs to different plant tissues. We assessed the biological effects by the evaluation of biomass accumulation (cell culture experiment), germination tests, and phenotypical analysis of germinated seedlings. The detection of CBNs inside seeds was performed by Raman spectroscopy and TEM imaging. The expression of aquaporin-encoded genes was analyzed by RT-PCR and gene expression was achieved by microarray analysis (figure 2).
Figure 2. Schematic diagram illustrating the experimental design of the presented study. The length of the nanomaterials was measured using TEM and compared to manufacturer data.
Download figure:
Standard image High-resolution image3.2. The biological response of tobacco cells on the application of CBNs
The in vitro experiment was performed using tobacco callus culture. This model culture is a fast-grown callus culture for plant species of the Solanaceae family [40]. The tobacco callus was exposed to four different CBNs at two working concentrations (50 and 100 μg ml−1). All results were compared to callus growing on control media (MS) or callus exposed to AC (negative control). After 30 days of incubation, the tobacco callus culture was collected and weighed. At 50 μg ml−1 concentration, all CBN-exposed calli indicated a significant increase of fresh biomass by 35% (helical MWCNTs), 33% (graphene), 46% (long MWCNTs), and 22% (short MWCNTs) (figure 3). After drying the callus overnight, the measured dry biomass of callus exposed to all CBNs remained significantly higher than the control (figure 3(c)). Helical MWCNTs, graphene, long MWCNTs, and short MWCNTs increased cell biomass by 35%, 30%, 12%, and 13% respectively, compared to the control. The addition of AC at a concentration of 50 μg ml−1 to the growth media also increased the fresh biomass (22%) and the dry biomass (10%) compared to the control. AC at a higher concentration (100 μg ml−1) significantly decreased the growth of the tobacco callus (fresh and dry biomass) (figures 3(b), (c)). However, the fresh biomass of the callus was significantly increased by 63% and 71% when cells were exposed to helical and long MWCNTs, respectively, at 100 μg ml−1 concentration (figure 3(b)). The dry weight of the callus exposed to long (37%) and short (24%) MWCNTs (100 μg ml−1) was also significantly higher than the control (figure 3(c)).
Figure 3. CBNs induce tobacco callus culture growth. (a) Tobacco callus culture exposed to control media (Control), activated carbon (AC), helical MWCNTs (Helical), graphene, long MWCNTs (Long), and short MWCNTs (Short) at 50 μg ml−1 for 30 days. (b) Accumulation of fresh biomass after 30 days of cultivation on medium with or without CBNs. (c) Accumulation of dry biomass after 30 days of cultivation on medium with or without CBNs. * p < 0.05 using the Tukey post-ANOVA test, CBNs compared to control. Error bars represent standard error values.
Download figure:
Standard image High-resolution imageWe have previously shown that MWCNTs were able to enhance the growth of callus culture (55%–64% increase over control) [12]. SWCNHs, another carbonaceous material with a spherical aggregate shape, were able to increase callus growth up to 78% [13]. While both nanomaterials had different morphologies, they influenced the proliferation of tobacco callus cells and significantly increased its biomass accumulation. In agreement with these reports, all CBNs used in this study positively impacted on the growth of the tobacco callus culture (figures 3(b), (c)). Long MWCNTs showed the highest impact with an increase of fresh biomass of 46% at 50 μg ml−1 concentration and 71% at 100 μg ml−1 concentration, compared to the control. Helical MWCNTs were the second-best nanomaterials with an increase of fresh biomass of 35% at 50 μg ml−1 concentration and 63% at 100 μg ml−1 concentration, compared to the control. Graphene and short MWCNTs had a lower impact compared to long and helical MWCNTs on tobacco cell growth.
The modest activation of growth in the callus, when exposed to AC, was not surprising. We noticed early on [12] that AC activated growth of the tobacco cell culture (16% of activation) in low concentrations (5 μg ml−1) but caused toxicity at higher doses (100; 500 μg ml−1). Conversely, the MWCNTs used in the previous study [12], even in high doses (500 μg ml−1), were not toxic for the tobacco callus. We believe that the mechanisms of activation of cell growth caused by AC and by nano-sized CBNs are different. It has been suggested in the literature that the absorbent nature of this material allows the removal of toxic substances that could have a negative influence on the growth of the callus [41]. The exact mechanism of the positive effects of CBNs in planta is unknown. However, we have noticed that MWCNTs [31] and SWCNHs [2] can influence the expression of a number of stress-related genes that were not affected by the application of AC. Here, we observe that all four tested CBNs played a positive role in the stimulation of tobacco cell growth. The differences in the levels of activation of cell growth between tested CBNs can be associated with differences in morphology of CBNs that may allow for better penetration of plant cells. However, we cannot exclude that the presence of different amounts of metal impurities in types of used CBNs can impact on the proliferation of plant cells as well [42–44].
The assessment of CBN interaction with plant cell culture is an important part of understanding whether the morphology of the carbonaceous material could play an important role at the plant cellular level. We notice here that all tested CBNs can be effective for the stimulation of tobacco cell growth. The comparison of the effect of four tested CBNs on fresh callus accumulation allowed us to notice that the longer the size of the CBNs applied to the cells, the higher the detected biomass increase (figure 3). The intensity of the effect of CBNs on fresh biomass can be roughly exhibited in the following sequence (long MWCNT > helical MWCNT > graphene > short MWCNT). It is interesting that we cannot observe a direct link between the increase of cell biomass and size of the outer diameter of the specific shape of the used CBNs. In order to better understand whether there is an association between the specific shape of CBNs and the biological effect at plant organismal level, we tested the seed germination and plant development of tomatoes in response to four different-shaped CBNs used in the previous cell culture experiment.
3.3. The biological response of tomato seeds and young seedlings on application of CBNs with different morphologies
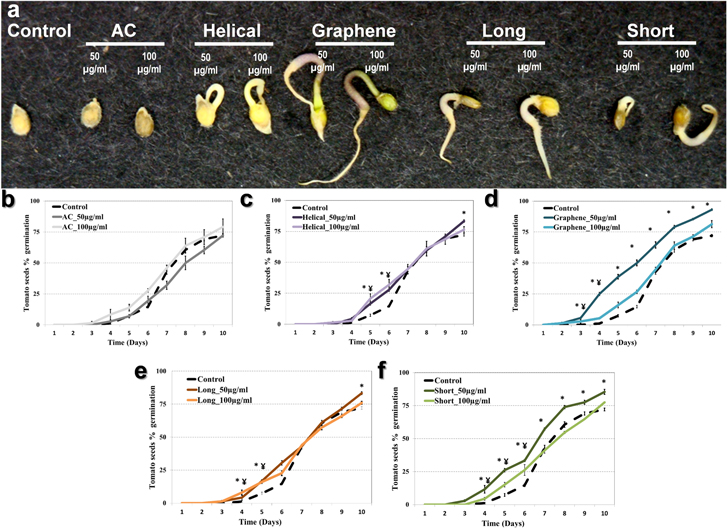
To test whether the morphology of applied CBNs can be linked to a specific bioresponse of plants, tomato seeds were sterilized and placed on an MS medium containing four types of CBNs at concentrations of 50 and 100 μg ml−1. Media without CBNs, as well as media containing AC, were used as controls. Germination was recorded by monitoring the appearance of the first root. Figure 4(a) shows that the exposure of seeds to all tested CBNs accelerated the process of germination compared to the control.
Figure 4. Germination of tomato seeds exposed to CBNs through the introduction of CBNs in agar growth medium. Different CBNs were added in MS medium in concentrations of 50 and 100 μg ml−1. (a) The phenotype of tomato seeds after five days of CBN exposure. (b)–(f) Percentage (%) of germination of tomato seeds exposed to activated carbon (AC), helical MWCNTs, graphene, long MWCNTs, and short MWCNTs, respectively. Each figure shows the exposure of seeds to CBNs at a concentration of 50 μg ml−1 and 100 μg ml−1. Results are shown as an average of measurement of 72 seeds per each condition. * p < 0.05 using the Tukey post-ANOVA test, 100 μg ml−1 compared to control; ¥ p < 0.05 using the Tukey post-ANOVA test, 50 μg ml−1 compared to control. Error bars represent standard error values.
Download figure:
Standard image High-resolution imageOverall, seeds exposed to CBNs germinated faster and at a higher rate compared to control seeds. Indeed, it took three days for 8% of seeds exposed to graphene (50 μg ml−1) to germinate (figure 4(d)). On the other hand, 8% of seeds exposed to control media (no CBNs) started germinating at day 5 post-exposure. However, on the same day, 39% of seeds exposed to graphene (50 μg ml−1) and 23% of seeds exposed to helical MWCNTs (100 μg ml−1) had already germinated (figures 4(c) and (d)). Seeds exposed to short and long MWCNTs germinated on day 4 post-exposure with 8% and 6% germination rate, respectively (figures 4(e), (f)). After 10 days of observation, tomato seeds exposed to CBNs (50 μg ml−1) were found to have a higher germination rate (%) compared to control seeds (p < 0.05). At the highest concentration of CBNs (100 μg ml−1) we observed an early start of seed germination, but not an overall higher germination rate (figures 4(c)–(f)). The application of AC to seeds did not result in an early seed germination or an increase of the germination rate. The influence of nanomaterials (metal- or carbon-based) on seed germination has been discussed in a number of research papers [2, 8, 12, 13, 22, 30, 37, 45–48]. It is clear now that the shape/size of the carbon-based nanomaterial is not a critical factor in achieving positive effects in planta. However, some CBNs can be more effective than others. Here, we show that graphene and short MWCNTs had the greatest impact on the germination of tomato seeds. Moreover, the dose of applied CBNs used in germination tests plays a critical role in the observed bio-effect. We continued monitoring the development of tomato seedlings germinated on control medium and medium with four types of CBNs (figure 5).
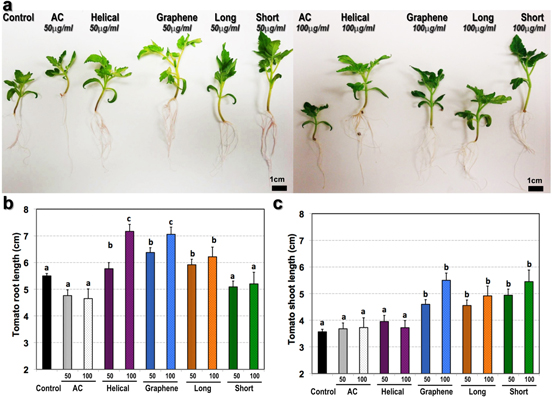
Figure 5. Growth characteristic of 20 day old seedlings germinated from seeds exposed to CBNs included in the medium. (a) The phenotype of representative 20-day-old seedlings. The total length of the root system (b) and major shoot (c) of control and CBN-exposed tomato seedlings. Tomato seedlings were germinated on medium supplemented with CBNs and grown on medium supplemented with CBNs at 50 μg ml−1 (50) and 100 μg ml−1 (100). Results are shown as an average of measurement of 48 seedlings per condition. The different letters means statistically different groups (p < 0.05) using the Tukey post-ANOVA test. Error bars represent standard error values.
Download figure:
Standard image High-resolution imageAt day 20 post-exposure, seedlings were collected for phenotypic measurement (figure 5, S2). The root length is the major phenotypical trait which is associated with plant response to the toxic agent. The average root lengths of seedlings exposed to helical MWCNTs (50 μg ml−1), graphene (50 μg ml−1 and 100 μg ml−1), and long MWCNTs (50 μg ml−1 and 100 μg ml−1) were significantly higher compared with control seedlings (figure 5(a)). Seedlings exposed to short MWCNTs did not show any significant difference in root length compared to the untreated seedlings. The shoot length of CBN-exposed seedlings was significantly increased in response to all applied CBNs except helical MWCNTs (figure 5(b)). Graphene and short MWCNTs at 50 μg ml−1 concentration had the highest impact on shoot length compared to other applied CBNs (figure 5(c)). Both phenotypical traits (shoot length, root length) were similar to control when seedlings were exposed to AC (negative control). Figure S2(a) demonstrates that fresh root biomass was increased for all CBN-treated roots compared to control (untreated roots) (p < 0.05). It is interesting that the total dry biomass of CBN-treated roots was not increased with the exception of roots treated with short MWCNTs (figure S2(b)). We hypothesize that the observed enhancement of fresh root biomass can be associated with an increase of water uptake by CBN-treated roots. Similar to the root system, the fresh biomass of tomato shoots was significantly affected by the treatment of CBNs (figure S2(c)). Roots treated with graphene and short MWCNTs at 50 μg ml−1 produced the highest fresh total biomass. No biomass increase was noticed for roots treated with helical MWCNTs. The shoot dry biomass increase was in direct correspondence with the increase of fresh shoot biomass (figure S2(b)).
3.4. Detection of CBNs in tomato seeds using TEM and Raman spectroscopy
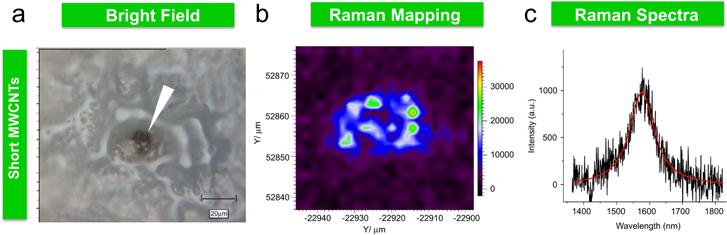
The ability of CBNs to penetrate seed coats was demonstrated previously for long MWCNTs [13] and SWCNHs [2]. For example, MWCNTs were able to penetrate the seed coat of barley, soybean, and corn [13]. Here, we demonstrate that there is a similar ability for one more type of tubular CBN to penetrate the seed coat of tomato seeds. Particularly, we selected short MWCNTs as tested CBNs with the highest ability to stimulate tomato seed germination (figure 4). By using Raman spectroscopy, we intended to confirm the link between the uptake of CBNs by endosperm and observe the effect of activation of germination of exposed seeds. In the experiment tomato seeds were incubated for 24 h on a medium supplemented with short MWCNTs at 50 μg ml−1, washed carefully and then sliced. All Raman mappings were focused inside the endosperm area of the seed (figure 6). Through the series of preliminary experiments (above) we found that the most optimal (in terms of signal to noise and minimal background luminescence) condition for the detection of carbon nanomaterials was achieved with 633 nm excitation. First, the microtomed slice of seed was mapped at millimeter scale with a low-resolution objective (x10) using image-stitching software to identify specific features of the sample, for easy navigation around the sample under higher magnification. Next, the higher magnification photomapping was done to identify possibly resolved darker areas on the seed within 100 um area. An optical image of the microtomed slide of the seed with short MWCNTs is shown in figure 6(a). To further improve the resolution of MWCNTs we moved to higher objective magnification. We noticed that the photoluminescence background was present in all samples with all excitation wavelengths. Moreover, in the area where short MWCNTs were detected the fluorescence background was even higher. To demonstrate the effect of the nanotubes on autofluorescence of the sample, we integrated Raman spectra between 1000–1700 cm−1. The map of integrated fluorescence intensity is shown in figure 6(b). The location of short MWCNTs appears on photo images as darker areas. In figure 6(a), the same area also exhibits much smaller autofluorescence. Raman spectra of the dark area are shown in figure 6(c). However, the area located in close proximity to the large aggregate of short MWCNTs shows enhanced autofluorescence. A possible mechanism of reduced autofluorescence originates in the quenching of luminescence by the conducting nature of the carbon nanotubes. The enhancement of autofluorescence in the proximity of short MWCNTs, on the other hand, may stem from the electric-field enhancement on the tips of the nanotubes comprising the aggregate. We tested this concept in other areas of the sample and confirmed that some regions indeed demonstrate enhanced autofluorescence, while the Raman spectra were not observed in all areas. Thus, enhanced autofluorescence and Raman maps are complimentary and can be used for the detection of MWCNTs in seed endosperm.
Figure 6. Detection of short MWCNTs in exposed tomato seeds using Raman spectroscopy. (a) Photographs of the selected area of the exposed endosperm taken with 50 × objectives. (b) Autofluorescence map of the area derived from the integration of the broad feature in the Raman spectrum from 1400 to 1700 cm−1. Note the low intensity of autofluorescence, where the dark area associated with an aggregate of short MWCNTs is observed. The high intensity of autofluorescence is associated with the antenna-like behavior of nanotubes, where the high density of the electrical field is expected at the ends of their tips. The Raman spectrum of short MWCNTs is most pronounced in the area of the sample with the lowest autofluorescence. Note the G-band (1580 cm−1) and the corresponding map of the integrated intensity of the MWCNT Raman band, 1450–1700 cm−1 (c). The highest intensity was observed where the autofluorescence is the lowest.
Download figure:
Standard image High-resolution imageOur results confirmed again that Raman spectroscopy is a powerful instrument for the detection of carbon nanotubes inside plant tissues. However, big aggregates of CBNs can be found in seeds by transmission electron microscopy (TEM) as well. We performed TEM for seeds exposed to long MWCNTs. Thus, endosperm of tomato seeds exposed for 24 h to the long MWCNTs was sliced and visualized using TEM. We observed that many black aggregates were present inside the endosperm of seed exposed to long MWCNTs while they were absent in the control seed (figure 7). The detection of CBNs with TEM is difficult due to the nature of living tissue composed mainly of carbon elements. However, both methodologies (Raman spectroscopy and TEM) used in this study are complementary and very informative about the ability of different types of CBNs to penetrate the seed coat and reach the endosperm.
Figure 7. TEM detection of long MWCNTs in exposed tomato seeds. Tomato seeds were exposed to long MWCNTs for 24 h, carefully washed, dried by filter paper and cut longitudinally. Control seeds (a) and seeds exposed to long MWCNTs (b) were prepared for TEM as described in section 2.
Download figure:
Standard image High-resolution image3.5. Helical MWCNTs affect total transcriptome of tomato seeds and seedlings
Recently, we analyzed the total transcriptome in plant organs (tomato model system) exposed to two different types of CBN (long MWCNTs [31] and SWCNHs [2]). We found that both types of CBN affected a number of genes involved in cellular responses and stress signaling. Thus, many genes involved in the response of plants to pathogen attacks were differently regulated in CBN-treated tomato organs. We also noticed that the key genes involved in plant–water relations (aquaporins) were upregulated in tomato roots [23, 31] and in seeds of soybean, corn, and barley [13]. Such comparative gene expression studies can help clarify whether all CBNs share a similar mechanism of activation of seed germination. Here, we performed genomic studies using helical MWCNTs, which have a different shape from the rest of the CBNs used for previous genomic studies (long MWCNTs, SWCNHs). To prove that CBNs with a wide range of shapes may affect plant transcriptome in a similar manner, we carried out a microarray analysis (Affymetrix platform) of tomato seeds exposed to helical MWCNTs for 24 h and seedlings exposed to helical MWCNTs for 11 days. The microarray analysis of seeds and seedlings exposed to helical MWCNTs (25 μg ml−1) revealed that the expression of a number of genes had been altered. The total number of transcripts affected (up- and downregulated) by the exposure of helical MWCNTs to tomato seeds was 79. The number of transcripts with known functions was 15, from which four transcripts were downregulated, and 11 others were upregulated (figure 8). It was noticed that proline dehydrogenase (LesAffx.3568.1.S1_a_at), an enzyme involved in reproduction and response to water stress [49], was upregulated. This gene was also found to be involved in pathogen defense in Arabidopsis [50]. We have previously suggested that carbon nanoparticles can be sensed by plants as pathogens at the level of the transcriptome [31]. In the current study, pathogenesis-related protein P2 (Les.4345.1.S1_at) was two-fold upregulated compared to control (figure 8). Several other genes related to the response to abiotic stimulus were upregulated including TDR3 protein (Les.49.1.S1_at) and (ERT 1b) ripening-related mRNA (Les.424.1.S1_at). The transcript profiling of seedlings exposed to helical MWCNTs for 11 days has also shown significant differences compared to tomato seedlings growing on control growth media (figure S3). A total of 474 genes were significantly altered (p < 0.01); among them, 111 genes were annotated. Figure S3 is a heat map of the genes with known functions showing that 38 genes were upregulated, and 73 others were downregulated. Among the known upregulated genes in seedlings, we observed that most of transcripts were involved in the response to endogenous stimulus (Les.3818.1.S1_at, Les.3809.2.S1_a_at, Les.3751.1.S1_at, Les.4101.2.S1_a_at, Les.3702.1.A1_at, Les.3679.1.S1_at, Les.274.1.S1_at, Les.3573.1.S1_at, Les.4302.1.S1_at, Les.3730.1.S1_at, Les.3971.2.S1_a_at, Les.141.1.S1_at, and Les.3733.1.S1_at).
Figure 8. Heat map of transcripts of significantly affected genes in tomato seeds exposed to helical MWCNTs (only genes with known functions are shown). Three biological replicates were used for control seeds (WT) and helical MWCNT exposed seeds (Helical). Hierarchical clustering (Euclidian distance) of log2 fold changes over average control are shown.
Download figure:
Standard image High-resolution imageUsing the tomato functional genomics database, the genes affected by the application of helical MWCNTs were categorized based on their specific biological processes (table S1). We found that three major processes were affected by helical MWCNTs in seeds: cellular processes, metabolic processes, and response to an abiotic stimulus. In seedlings, the three major processes altered by helical MWCNTs also belong to cellular and metabolic processes and responses to stress. Such results are in good correspondence with our previous microarray data established using tomato plants exposed to regular MWCNTs [31] and SWCNHs [2]. The differences in transcript abundance were confirmed by RT-PCR (figure S4). The Les.50.1.S1_at ribonuclease gene was upregulated in microarray analysis of exposed tomato seeds (figure 8). The upregulation of Les.50.1.S1_at was confirmed by RT-PCR (figure S4(a)). Les.3635.1S1_at Subtilise-like protease and Les.1841.1.S1_at 1-aminocyclopropane-1-carboxylate synthase were upregulated in the exposed seedlings as noticed by microarray analysis. This observation was also confirmed by RT-PCR (figures S4(b) and (c)).
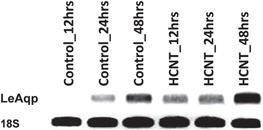
The early germination of tomato seeds exposed to different CBNs including helical MWCNTs is an intriguing phenomenon [1, 12]. The ability of CBNs with different morphologies to penetrate the seed coat at an early stage of exposure raises questions about the mechanism of the positive effect of CBNs on seeds. The uptake of water is the initial and major step of seed germination [51]. In our previous studies, we documented that MWCNTs can activate the expression of water channel genes (aquaporins) and may play a role in the enhancement of water uptake during seed germination [13]. To prove that CBNs other than typical carbon nanotubes (MWCNTs) can also activate the water channel gene in seeds, we performed RT-PCR analysis of the expression of the tomato aquaporin (LeAqp) gene in seeds exposed to helical MWCNTs. LeAqp gene expression was determined in a real-time manner at 12, 24, and 48 h after exposure to helical MWCNT supplemented medium or controlled medium (figure 9). The RT-PCR analysis proved that the LeAqp gene was upregulated in exposed seeds compared with control seeds at each specific time point. In fact, it took less than 12 h to see a slight upregulation of aquaporin genes in exposed seeds, while the control did not show any sign of the gene expression at this time point.
Figure 9. RT-PCR analysis of expression of the tomato aquaporin gene (LeAqp) in tomato seeds growing on a medium supplemented with helical MWCNTs. The expression of aquaporins was detected at three time points (12, 24, and 48 h). 18S rRNA was used as an internal control.
Download figure:
Standard image High-resolution imageEstablished genomics results reveal some evidence that CBNs with tubular structures can not only affect the physiology of plants and plant cells in similar ways, but may also share common molecular mechanisms of the observed effects.
4. Conclusion
The early documented positive effect of CBNs with tubular structures (single-walled and multi-walled nanotubes) [13, 31] and SWCNHs [2] on seed germination and cell/plant growth raised a valid question about the links between the morphology of CBNs and the response of exposed plants. In this work, we investigated whether the positive effect on germination and plant growth can be achieved by wide range of CBNs including those with a 2D structure (graphene), nanotubes with a helical nature, and carbon nanotubes with different lengths (short or long multi-walled carbon nanotubes). In order to confirm the potential use of CBNs as affordable plant growth regulators, we intentionally used commercially available CBNs in the current study. Our data confirmed that CBNs with different morphologies have the ability to stimulate germination and cell growth. All tested CBNs can be used as regulators of growth of plant cell cultures. Under both tested concentrations of CBNs (50, 100 μg ml−1) biomass accumulation was significantly increased (12%–46%) when various CBNs were added into the medium. Similarly, all tested CBNs enhanced the germination of exposed tomato seed and growth of germinated tomato seedlings. The data of gene expression assays provided additional evidence that different CBNs may share a similar biological mechanism of positive effects in planta. Taking into account the documented overexpression of water channel genes in response to MWCNTs [13, 23, 31], SWCNHs [2] and helical carbon nanotubes (current study), we can conclude that CBNs with various morphologies can affect the water uptake in CBN-exposed plant organs. Comprehensive understanding of the mechanisms of the biological effects of CBNs will require more molecular and biochemical studies.
Acknowledgments
TEM (JC) and Raman (INI) experiments were conducted at the Center for Nanophase Materials Sciences, which is a DOE Office of Science User Facility. We thank Mrs Valerie K Lapham (Center for Electron Microscopy, North Carolina State University) for the preparation of samples of seeds for TEM.