Abstract
Single-walled carbon nanotube (SWNT) films are a potential candidate as porous conductive electrodes for energy conversion and storage; tailoring the loading and distribution of active materials grafted on SWNTs is critical for achieving maximum performance. Here, we show that as-synthesized SWNT samples containing residual Fe catalyst can be directly converted to Fe2O3/SWNT composite films by thermal annealing in air. The mass loading of Fe2O3 nanoparticles is tunable from 63 wt% up to 96 wt%, depending on the annealing temperature (from 450 °C to 600 °C), while maintaining the porous network structure. Interconnected SWNT networks containing high-loading active oxides lead to synergistic effect as an anode material for lithium ion batteries. The performance is improved consistently with increasing Fe2O3 loading. As a result, our Fe2O3/SWNT composite films exhibit a high reversible capacity (1007.1 mA h g−1 at a current density of 200 mA g−1), excellent rate capability (384.9 mA h g−1 at 5 A g−1) and stable cycling performance with the discharge capacity up to 567.1 mA h g−1 after 600 cycles at 2 A g−1. The high-loading Fe2O3/SWNT composite films have potential applications as nanostructured electrodes for various energy devices such as supercapacitors and Li-ion batteries.
Export citation and abstract BibTeX RIS
Introduction
Carbon nanotubes (CNTs) have been extensively explored in building porous conductive electrodes for energy storage systems such as supercapacitors and lithium ion batteries (LIB) [1–5]. In most applications, the main function of CNTs is to provide a conductive substrate or skeleton for charge carrier transport. Various active materials (e.g. pseudo-polymers, oxides, metallic catalysts) must be introduced into the CNT network to promote redox reactions or Li-ion intercalation process and thus to improve specific capacity [4–6]. In this regard, the distribution and mass loading of active materials are very important and directly determine the ultimate performance of those hybrid electrodes [7, 8]. In general, a large mass loading of nanoscale particles with uniform distribution is favorable for achieving high performance. However, it remains challenging to fabricate high-loading hybrid structures since those introduced nanoparticles tend to aggregate at increased loading and consequently reduce available active sites.
Among various transitional metal oxides, Fe2O3 has been considered as a potential anode material owing to its low cost, abundance, being environmentally benign and high theoretical specific capacitance (1007 mAh g−1) [9–12]. In order to tackle the issues of inferior cycling stability and low intrinsic conductivity of Fe2O3, many strategies including synthesis of tailored nanostructures and design of hybrid materials on nanoscale have been explored. Hollow Zn-doped Fe2O3 nanospheres (200–500 nm) exhibited an initial capacity of 861.5 mAh g−1 (1 A g−1), and 580 mAh g−1 after 500 cycles [13]. Nanoplates of α-Fe2O3 with a thickness of 20–30 nm and a side length of 100–300 nm were embedded in graphene networks, and the resulting Fe2O3/graphene composite demonstrated discharge capacities of 896 mAh g−1 up to 200 cycles at 5 C and 429 mAh g−1 up to 1000 cycles even at a 10 C rate [14]. A hybrid material of CNT-encapsulated Fe2O3 nanoparticles was prepared by immersing CNTs with open ends into a Fe(NO3)3 solution followed by thermal decomposition, and the Fe2O3-filled CNTs delivered a reversible capacity of 811.4 mA h g−1 after 100 cycles at a current density of 35 mA g−1 and high rate capability [15]. Recently, by oxidizing as-grown single-walled carbon nanotube (SWNT) films containing residual catalyst, freestanding Fe2O3/SWCNT hybrid membranes were prepared and directly used as LIB anodes [16, 17]. In the first report, a high reversible capacity of 1243 mAh g−1 (at 50 mA g−1) was obtained [16], while the second work tested long-term stability with a reversible capacity of 375.5 mAh g−1 over 800 charge/discharge cycles at a rate of 3 A g−1 [17]. Both studies acquired relatively high Fe2O3 loadings (88.0 wt% and 63.4 wt%, respectively) in the SWNT membranes, which was considered as a critical factor for achieving high specific capacity. When even higher Fe2O3 loading was attempted (e.g. 97 wt%), serious aggregation occurred and the membrane structure was destroyed [16]. Although hybrid structures made by grafting metal oxides onto CNTs or graphene have been studied extensively, there still an imperative need for a simple, efficient fabrication process toward pre-designed hybrid electrodes with well controlled micro-structure. Specifically, for the SWNT-Fe2O3 hybrid films which are made by direct annealing to expose residual Fe catalyst particles, it remains unclear whether the oxide morphology and loading (particularly for reaching high mass loadings) could be tailored systematically, and the influence of these important parameters on the electrochemical behavior. Understanding the structure-performance relationship and the underlying mechanism is essential for developing high performance hybrid electrodes and energy storage systems.
Here, we present a simple and controllable method to produce Fe2O3/SWNT composite with tailored mass loading and uniform distribution of Fe2O3 nanoparticles among a highly conductive SWNT network. We utilize the residual catalyst (Fe) in as-grown SWNT films and directly convert it into Fe2O3 by annealing at predefined temperature. The content of Fe2O3 is tuned up to 96 wt% at an annealing temperature of 600 °C. When used as a porous electrode for lithium ion intercalation, the Fe2O3/SWNT composite films show high reversible capacity (1007.1 mA h g−1 at 200 mA g−1), excellent rate capability (384.9 mA h g−1 at 5 A g−1) and stable cycling performance with the discharge capacity up to 567.1 mA h g−1 after 600 cycles at 2 A g−1.
Experimental methods
Preparation of Fe2O3/SWNT composite films
The SWNT films were continuously synthesized by using chemical vapor deposition (CVD) technique, and was blown out to low-temperature zone by mixed Ar/H2 as the carrier gas. And then the SWNT films were collected and twisted on a rectangular metal frame for 30–60 min. As grown SWNT films were subjected to thermal annealing at set temperatures of 450 °C, 500 °C, 550 °C or 600 °C for 1 h in air, respectively, to obtain Fe2O3/SWNT composite films with different Fe2O3 loading percentages which were then applied as electrode materials in LIB.
Characterizations
The SWNT or Fe2O3/SWNT composite films were characterized by Scanning electron microscopy (SEM) (JEOL JSM-6700F) and TEM (JEOL JEM-2100). The crystalline phase of the samples was determined on a x-ray diffractometer (XRD) using a PANalytical X'Pert Powder instrument with Cu Ka radiation. Raman spectrometer (Renishaw-in Via Reflex) was obtained with the laser wavelength λ = 514 nm. Thermogravimetric analysis (TGA) was carried out using Linseis STA PT1600 at a heating rate of 10 °C min−1 from room temperature to 1000 °C in air. The x-ray photoelectron spectroscopy (XPS) data was recorded on an ESCALAB250Xi apparatus at base pressure of 1 × 10−9 mbar with an Al Kα excitation source. The N2 adsorption/desorption isotherms and pore size distributions were measured to investigate the specific areas and porous nature of the Fe2O3/SWNT composite films by using the NOVA 4200e at 77 K.
LIB measurement
LIBs performance was evaluated using CR 2032 type coin cells assembled in an argon-filled glove box. A piece of wafer-shaped free-standing Fe2O3/SWNT composite electrode was assigned as the working electrode without any addition of carbon and binder. The average mass loading of each piece of the Fe2O3/SWNT electrode used in the experiment was shown in table S2. Celgard 2400 was used as a separator. Li foil was used as the counter electrode. The electrolyte consisted of a solution of LiPF6 (1 M) containing vinylene carbonate (2 wt%) in ethylene carbonate/dimethyl carbonate/diethyl carbonate (1:1:1, volume ratio). The cycle life and rate capability of the cells were tested on a battery test system (Neware, CT 3008 W) within a fixed voltage window of 0.05–3.000 V. Cyclic voltammetry measurement was conducted at 0.1 mV s−1 within the range from 0.02 to 3.0 V by an electrochemical workstation (CorrTest CS2350). Electrochemical impedance spectroscopy measurement was performed at frequency range from 100 kHz to 0.01 Hz at the identical electrochemical workstation. The theoretical capacity (C) of the hypothetical mixture of Fe2O3/SWNT is calculated based on the total mass of the electroactive materials containing both Fe2O3 and SWNT.
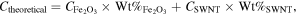
where C is the theoretical specific capacity of each component in the composite, Wt% is the percentage of the components in the composite.
Results and discussion
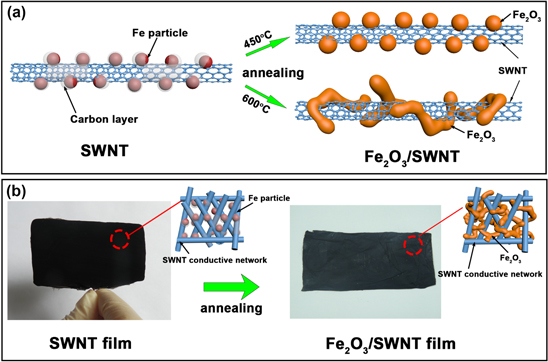
Figure 1(a) illustrates the synthesis process of Fe2O3/SWNT composite films, in which residual Fe catalyst particles attached on SWNT bundles are converted to Fe2O3 with different shapes and loadings by thermal annealing at selected temperature. First, continuous pristine SWNT films were synthesized by the catalytic CVD with xylene/ferrocene as the carbon precursor and catalyst [18, 19], in which a higher ferrocene concentration (0.05 g ml−1) was adopted to introduce extensive iron nanoparticles accompanying the growth of SWNTs. The as-grown SWNT films were collected by a rotating metal wire frame layer by layer, and were loosely stacked into a porous membrane with a black and textile-like surface, at a thickness of ∼2 mm (figure 1(b)). Second, the as-collected SWNT membrane was heat treated for 1 h in air atmosphere at predefined temperatures from 450 °C to 600 °C. After annealing, the membrane color changed from black to light yellow, indicating that the Fe particles have been oxidized. There is a condensation effect at the same time, as the membrane thickness decreased from 2 mm to 15 μm much like a thin paper sheet.
Figure 1. Fabrication of Fe2O3/SWNT composite films. (a) Illustration of the as-grown SWNT films consisting of residual Fe particles grafted on SWNTs, and the annealing process which converted Fe to Fe2O3 nanoparticles with different morphologies depending on the annealing temperature (450 °C or 600 °C). (b) Photos of an as-grown SWNT film (7 cm × 4 cm × 2 mm) collected on a metal frame and the resulting Fe2O3/SWNT composite film (6.5 cm × 3.5 cm × 15 μm) after annealing. Insets show the structural model of these two samples.
Download figure:
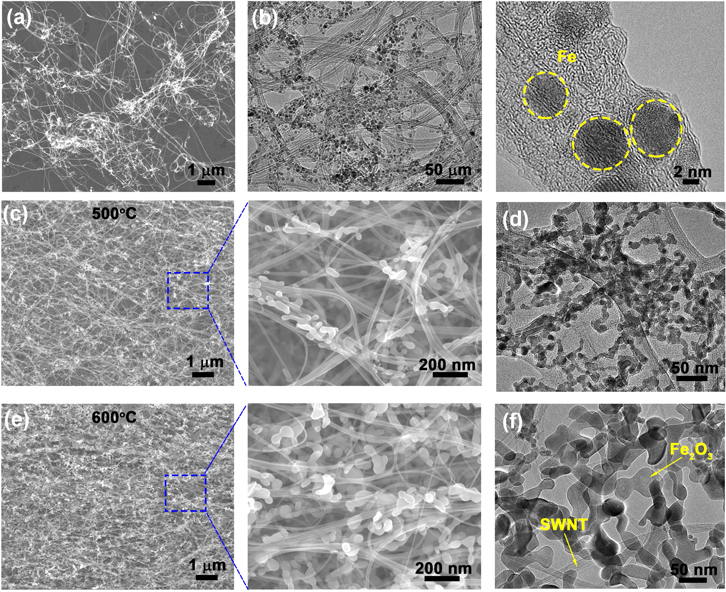
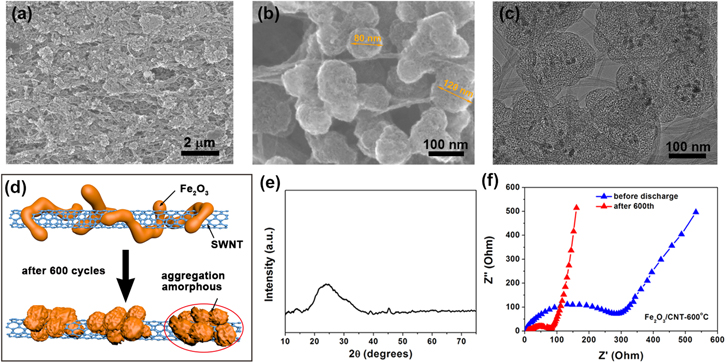
Standard image High-resolution imageSEM and transmission electron microscopy characterization reveals the morphology evolution of the Fe particles in pristine and annealed SWNT films. Small spherical Fe particles (diameters 5–10 nm) distribute uniformly throughout the SWNT network (figures 2(a), (b)). These are the residual catalyst formed during CVD process which usually attach to the surface of SWNT bundles; some particles are covered by thin amorphous carbon shells. After annealing at 500 °C, the Fe particles grow larger (due to oxidation) but are still well adhered to the SWNT network (figures 2(c), (d)). At even higher temperature (600 °C), the size of Fe2O3 particles further increases, and it can be seen that the porous space between SWNT bundles has been occupied by a large amount of particles (figure 2(e)). Although some SWNTs have been lost during annealing at 600 °C in air, the remaining SWNTs maintain their original continuous network which is critical for forming hybrid structures with good mechanical and electrical properties.
Figure 2. Structural characterization on SWNT and Fe2O3/SWNT films. (a) SEM image of SWNT film showing a lot of Fe nanoparticles dispersed uniformly throughout the SWNT network. (b) Low and high-magnification TEM images of the SWNT film clearly revealing Fe particles (dashed circles). (c), (d) SEM and TEM images of the Fe2O3/SWNT composite film annealed at 500 °C. (e), (f) SEM and TEM images of the Fe2O3/SWNT composite film annealed at 600 °C showing large Fe2O3 nanoparticles with changed morphology.
Download figure:
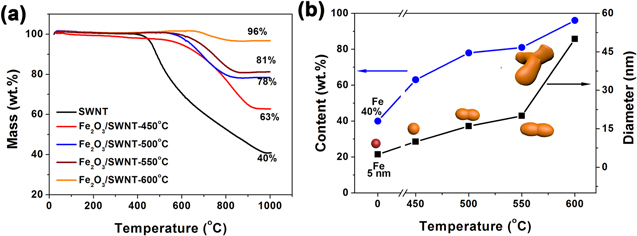
Standard image High-resolution imageThe weight percentages of those Fe and Fe2O3 nanoparticles were determined by TGA heating to 1000 °C in air (figure 3(a)). For the pristine Fe/SWNT film, a remaining weight of about 40 wt% was measured after complete burning of SWNTs, showing a considerable Fe content. The combustion of SWNTs mainly started when the temperature increased to above 400 °C, in which oxidation of Fe particles would occur. Thus, we have carried out a systematic study on the effect of annealing temperatures in the range from 450 °C to 600 °C. By performing the same TGA analysis on four annealed Fe2O3/SWNT films, the content of Fe2O3 could be determined to be 63 wt%, 78 wt%, 81 wt% and 96 wt% for composite films annealed at 450 °C, 500 °C, 550 °C and 600 °C, respectively (figure 3(b)). Further increasing the annealing temperature caused significant loss of SWNTs which is undesirable for ensuring structural integrity. Despite of that, our Fe2O3 loadings (up to 96 wt%) are much higher than previous reports (88.0 and 63.4 wt%) [16, 17].
Figure 3. (a) TGA curves (recorded from ambient temperature to 1000 °C in air) for the SWNT film and Fe2O3/SWNT composite films prepared by thermal annealing at 450 °C, 500 °C, 550 °C and 600 °C, respectively. (b) Calculated Fe2O3 contents and nanoparticle diameters in the SWNT film and those annealed at different temperatures. Insets show the nanoparticle shape evolution.
Download figure:
Standard image High-resolution imageTEM measurements on the Fe and Fe2O3 nanoparticles (figure 2 and supporting information, figure S1 is available online at stacks.iop.org/NANO/28/345703/mmedia) also show consistent increase of particle size from ∼5 nm to more than 50 nm at increasing annealing temperature (figure 3(b)). At the same time, the particle shape shows some variation, from tiny spheres (original Fe catalyst) to elongated columns and finally becomes a branched three-dimensional (3D) assembly. This shape evolution is due to the growth of Fe particles and the coalescence of adjacent particles (forming elongated columns) during annealing, and merging of several columns into a 3D structure at high temperature (e.g. 600 °C) (inset of figure 3(b)). However, such aggregation events are limited in a few adjacent nanoparticles, therefore the overall uniform distribution is retained in the resulting Fe2O3/SWNT film, which is important for later electrode applications.
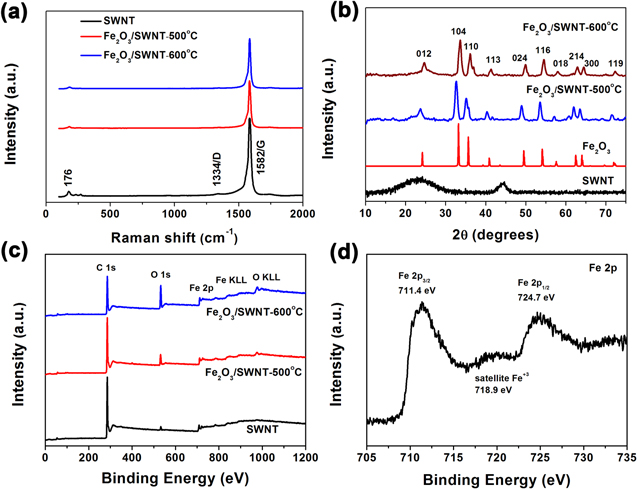
We have investigated whether the thermal annealing treatment affects the crystalline structure of SWNTs by Raman spectra (figure 4(a)). Both the SWNT and Fe2O3/SWNT composite films show a high G band (1582 cm−1) and very low D band (1334 cm−1), indicating high structural quality with negligible defect sites [20, 21]. Co-deposited amorphous carbon on Fe particles has been removed by oxidation, leading to reduced D band. The radial breathing modes uniquely related to SWNTs are still present (although weakened), in consistent with TEM observation. The crystalline structure of nanoparticles also has been studied by XRD analysis on the SWNT and Fe2O3/SWNT films, and pure Fe2O3 powders as a reference sample (figure 4(b)). In addition to the characteristic graphitic (002) and (101) diffraction peaks of SWNTs at 25° and 44° as seen in the pristine sample, more diffraction peaks coming from Fe2O3 emerge from the annealed films. These peaks correspond to reflections from (012), (104), (110), (113), (024), (116), (018), (214), (300) and (119) planes, which match those of the standard α-Fe2O3 sample (JCPDS card No. 33-0664) and thus identify a well crystallized and pure hematite structure. To further confirm the XRD results, chemical bonding states in the product are provided by XPS measurements (figure 4(c)). The XPS survey scan of Fe2O3/SWNT shows three key elements (C, O, and Fe) in the sample. The Fe 2p XPS spectra of the sample exhibit two peaks at 724.7 and 711.4 eV, corresponding to the Fe 2p1/2 and Fe 2p3/2 spin-orbit peaks of Fe2O3 (figure 4(d)) [22]. Moreover, a satellite peak appears at 718.9 eV, which is the characteristic of Fe2O3. The high resolution XPS C 1 s spectrum (figure S2a) ranging from 282 to 295 eV can be fitted to three components. The first component is found at 284.7 eV, and corresponds to sp2 carbon atoms, the second (at 285.8 eV), corresponds to sp3 carbon atoms, and the third peak located at 288.4 eV can be assigned to the carboxylic (COOH) or epoxy groups. Similarly, The peak at 530.4 eV in the O 1 s spectra (figure S2b) mainly fit the signals of metal oxide, Other O 1 s peaks at ∼531.7 and 532.9 eV indicate the existence of residual oxygen-containing groups [15, 23].
Figure 4. Structural analysis of SWNT and Fe2O3/SWNT composite films. (a) Raman spectra of the SWNT film and Fe2O3/SWNT films annealed at 500 °C and 600 °C, respectively. (b) XRD patterns of the Fe2O3/SWNT composite films, Fe2O3 and SWNT film. (c) XPS wide survey of the SWNT film, Fe2O3/SWNT-500 °C and Fe2O3/SWNT-600 °C composite films. (d) Fe 2p XPS spectra of the Fe2O3/SWNT-600 °C composite film.
Download figure:
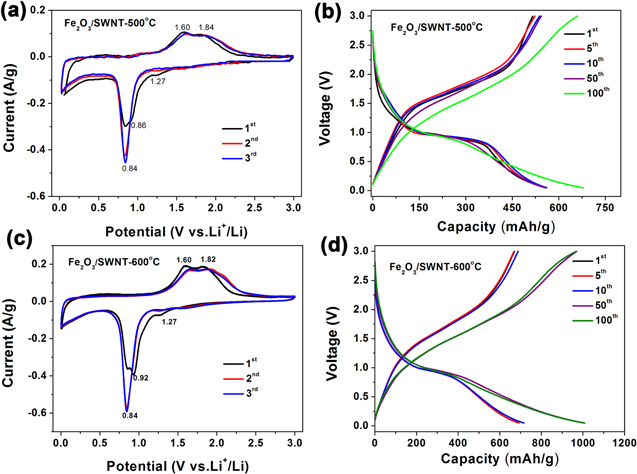
Standard image High-resolution imageMotivated by the hybrid structure of the Fe2O3/SWNT composite films where Fe2O3 nanoparticles are distributed uniformly among a conductive SWNT network, and that Fe2O3 is a host for Li-ion intercalation, we have evaluated their electrochemical lithium storage properties as an anode material for LIBs. Figure 5 compares the CV and galvanostatic charge/discharge curves between the Fe2O3/SWNT-500 °C and Fe2O3/SWNT-600 °C composite electrodes. Figures 5(a) and (c) present the CV for the first three Li-ion insertion/extraction cycles with a scan rate of 0.1 mV s−1 between a voltage window of 0.02 and 3.0 V. The curves of both samples show similar electrochemical characteristics, implying similar redox reactions during the initial charge/discharge processes. Two main cathodic peaks can be observed in the initial negative scan, where a first small peak at 1.27 V reflects the formation of LixFe2O3, and a sharp peak at 0.86 or 0.92 V represents the reduction of Fe3+ to Fe0 and decomposition of electrolyte which results in the formation of solid electrolyte interface (SEI) [24–27]. The positive scan shows a broad peak at 1.60–1.84 V, indicating the oxidation reaction of Fe0 to Fe2+ and Fe2+ to Fe3+, respectively. With more cycles, only one peak is observed for the cathodic process, indicating that an irreversible phase transformation occurred during lithium ion intercalation and extraction in the first cycle, the oxidation peak position and intensity remain almost unchanged, indicating good reversibility of the electrode reaction in the composite. According to previous studies, the detailed electrochemical reaction of Fe2O3 with respect to metallic lithium could be summarized as follows : Fe2O3 + 6Li+ + 6e− ↔ 2Fe0 + 3Li2O [13, 25].
Figure 5. Application as LIB electrodes. (a) CV curves of Fe2O3/SWNT-500°C composite film obtained at a voltage range of 0.02–3.0 V (versus Li+/Li) at 0.1 mV s−1. (b) Charge/discharge voltage profile of the Fe2O3/SWNT-500 °C at 200 mA g−1 for various cycles. (c) CV curves of Fe2O3/SWNT-600 °C composite film at 0.1 mV s−1. (d) Charge/discharge voltage profile of the Fe2O3/SWNT-600 °C at 200 mA g−1 for various cycles.
Download figure:
Standard image High-resolution imageFigures 5(b) and (d) show the charge/discharge curves of Fe2O3/SWNT composites in different cycles at a current density of 200 mA g−1. These profiles are consistent with the delithiation/lithiation processes involved in the redox reactions between the Li ions and iron oxide. The potential plateau in the range of 1.6–1.9 V corresponds to the Li ion insertion process, while that in the range of 0.7–1.0 V belongs to the Li ion extraction from iron oxide. For the Fe2O3/SWNT-600 °C composite electrode, the initial discharge capacity is 692.3 mAh g−1, and the successive charge capacity is 669.9 mAh g−1, with a coulombic efficiency of 103.3%. In the following 5th, 10th, 50th, 100th cycles, the voltage gap between charge and discharge potential plateaus becomes smaller with increasing cycle numbers, indicating facile reversible redox reaction [28]. The discharge and charge capacities increased to 1008.5 mAh g−1 and 966.2 mAh g−1 after 50 cycles at the current density of 200 mA g−1, respectively, with a coulombic efficiency of ∼104.3% (figure 5(d)). In comparison, the Fe2O3/SWNT-500 °C composite electrode exhibits a specific capacity of 678.6 and 659.5 mAh g−1 in the 100th discharge and charge process, which is lower than the capacity of the Fe2O3/SWNT-600 °C sample (figure 5(b)). The enhanced capacity of the sample annealed at higher temperature is due to more loading of Fe2O3 nanostructures (96 wt%) as characterized in figure 4. This result also indicates the importance of tailoring the active material loading in the electrode for maximum performance.
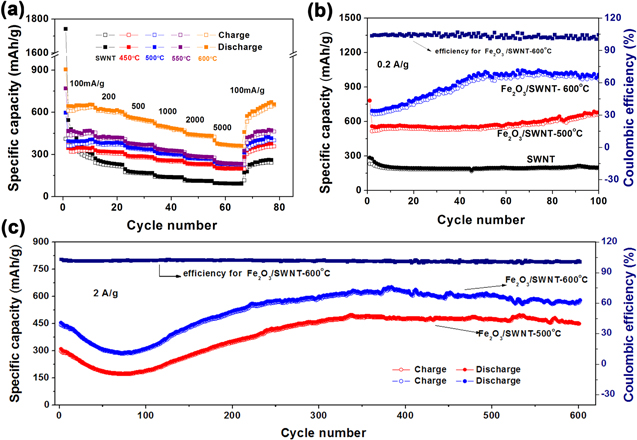
The rate performance of SWNT and Fe2O3/SWNT composite electrodes (annealed at four different temperatures) have been tested on assembled cells at different current densities (figure 6(a)). Specifically, after a high rate test at 5000 mA g−1, the reversible capacities of the Fe2O3/SWNT composites could return to their initial values at 100 mA g−1, demonstrating excellent high rate cycling characteristics. In particular, the Fe2O3/SWNT-600 °C composite shows much better electrochemical performance with the highest reversible capacity of 643.6 mAh g−1 at 100 mA g−1. Meanwhile, the capacity stabilizes at 384.9 mAh g−1 when the current rate increases to 5000 mA g−1 stepwise, nearly 60% of its capacity at 100 mA g−1. When restoring the current rate to 100 mA g−1, a reversible capacity of 651.9 mAh g−1 is recovered. The other three Fe2O3/SWNT electrodes show a similar trend during the rate capability test but their capacity values are lower than that of Fe2O3/SWNT-600 °C. For example, in the Fe2O3/SWNT-500 °C sample, the capacity is 231.8 mAh g−1 at 5000 mA g−1, nearly 58% of its capacity at 100 mA g−1. In contrast, there is a large capacity loss from 1741.0 to 543.2 mAh g−1 between the first and second cycles for the SWNT film (without annealing) because of the SEI formation on the electrode surface in the first discharge step [29, 30]. The capacity of the SWNT film decreases dramatically to 273.4 mAh g−1 after 10 cycles, and is only 94 mAh g−1 at 5000 mA g−1, showing a poor rate capability.
Figure 6. (a) Charge-discharge performance of the SWNT film and Fe2O3/SWNT composites (annealed at four temperatures) at various current densities (100–5000 mA g−1). (b) Charge-discharge voltage profile of the Fe2O3/SWNT-(500 °C, 600 °C) at 200 mA g−1 during 100 cycles. (c) Cycle performance of the Fe2O3/SWNT-(500 °C, 600 °C) and SWNT films at current densities of 2000 mA g−1 over 600 cycles.
Download figure:
Standard image High-resolution imageThe electrochemical cycling behavior of the SWNT and Fe2O3/SWNT composite electrodes are further evaluated by galvanostatic discharge-charge measurements at a current density of 200 mA g−1 in the potential range from 0.05 to 3 V (figure 6(b)), which shows very high cyclic stability of the Fe2O3/SWNT electrode. For the first lithiation/delithiation cycle, Fe2O3/SWNT-600 °C delivers a discharge capacity of 692.3 mAh g−1 and a charge capacity of 669.9 mAh g−1. The capacity increases steadily until 50 cycles, where the discharge capacity reaches 1007.1 mAh g−1 and the charge capacity is 969.1 mAh g−1, with 99.8% retention in the100th cycle, much higher than the SWNT film (200.3 mAh g−1) and Fe2O3/SWNT-500 °C (678.6 mAh g−1) at the same cycle. Although the contribution of the SWNT to the capacitance of the entire composite electrode is small, it plays an important role in the stability of the structure of the entire electrode. According to the BET data, the specific surface area of the Fe2O3/SWNT composite (450 °C, 500 °C, 550 °C, 600 °C)are approximately 422 m2 g−1, 335 m2 g−1, 206 m2 g−1, 159 m2 g−1, respectively. All the samples show a hysteresis loop in the P/P0 range of 0.8–1.0, and the pore width was around 32 nm. From the analysis on the porosity and pore structure of these films, it seems that the capacity of lithium batteries is not directly related by the pore structure (figure S3). Such phenomenon of the capacity increasing with cycling has been found in many iron oxides and other metal oxide anodes for LIBs. According to previous studies, this abnormal performance fluctuation is generally due to the formation of polymeric/gel-like films, which gradually proceeds in the active materials with a porous structure over more cycles [31–35]. In order to further confirm the durability of the Fe2O3/SWNT composite anodes to work at a higher rate (2000 mA g−1), their cyclability has been tested and the evolution of the specific capacity is shown in figure 6(c). The cyclic capacity profiles at 2 A g−1 until 600 cycles are reasonably good, remaining at ∼451.5, and 567.1 mAh g−1, for samples prepared at 500 °C and 600 °C, respectively. It is observed that the specific capacity of the electrode decreases during the first 70 cycles and then increases to a stable value. This phenomenon for iron-based and other transition metal oxide have been reported, the initial capacity fading is mainly because of the irreversible formation of the SEI films and decomposition of electrolyte at high charge/discharge current density, the subsequent capacity increase may be attributed to the improved Li-diffusion kinetics by the gradual activation process and the formation of the gel-like polymer layer with better electrolyte infiltration during cycling, which increase lithium storage [32, 34]. Furthermore, the cyclic capacity of Fe2O3/SWNT-600 °C at 1 A g−1 and 5 A g−1 until 250 cycles are remaining at ∼563.1 and 372.4 mA h g−1 (figure S4). A comparison with the electrochemical performances of the previous reports of the Fe2O3/carbon composite was shown in table S1.
To understand the microstructure evolution, SEM images of the Fe2O3/SWNT-600 °C composite after 600 cycles are provided (figure 7). Whereas the porous network and hybrid morphology remain there, the shape of Fe2O3 nanostructures has changed significantly. Individual nanoparticles have aggregated into bigger spherical clusters (with diameters of around 100 nm) although these clusters are still dispersed among the SWNT network (figures 7(a)–(c), figure S5). Thus, the microstructure evolution is characterized by two typical processes including (1) the change of crystalline structure to amorphous and (2) slight aggregation and formation of small clusters (as illustrated in figure 7(d)). In addition, the Fe2O3 nanoparticles have become amorphous, as verified by the XRD result (figure 7(e)). This result (complete loss of crystalline peaks in XRD) also indicates that virtually all Fe2O3 particles have participated into the Li-ion intercalation process, a full utilization of the high loading (96 wt%) active materials in our annealed SWNT films. It is interesting to see that although the Fe2O3 particles have become amorphous with slight aggregation due to the volume expansion/contraction upon Li insertion/extraction, the capacity remains high during 600 charge-discharge cycles. It means that those clustered Fe2O3 particles, which are still well attached to SWNT networks, could provide more active sites and defects available for Li-ion insertion, and this effect is found to contribute to the increase of capacity over cycles [36].
Figure 7. (a), (b) SEM images of the LIB electrode after 600 discharge-charge cycles for the Fe2O3/SWNT-600 °C composite film. (c) TEM image of electrode after 600 cycles for composite film. (d) Schematic of the structural evolution in the Fe2O3/SWNT-600 °C composite electrode after cycling tests. (e) XRD pattern of the Fe2O3/SWNT composite film after 600 cycles, in which the crystalline peaks of Fe2O3 have disappeared. (f) Nyquist plots of the Fe2O3/SWNT-600 °C sample before and after 600 cycles test.
Download figure:
Standard image High-resolution imageThe Nyquist plots of Fe2O3/SWNT-600 °C composite electrodes at different discharge/charge stages (before discharge and after 600th cycles) are exhibited in figure 7(f). Generally, the impedance spectra are composed of a semicircle at middle-high frequency and a straight sloping line at low frequency. The diameter of the semicircle can be assigned to the charge transfer resistance (Rct) and an inclined line at the low frequency region which represents the Warburg impedance related to the diffusion of Li ions in the electrode materials [37]. For the Fe2O3/SWNT-600 °C composite electrode, the resistance Rct decreases with cycling because of the formation of gel-like polymer layer, which can promote more sufficient contact between active materials and the electrolyte in this case, exhibiting enhanced and excellent long-life cycling performance.
Conclusions
In summary, free-standing Fe2O3/SWNT composite electrodes for rechargeable lithium batteries have been synthesized via a facile and controllable CVD method, in which the loading and morphology of active Fe2O3 can be tailored by the annealing temperature. When used as anode materials for LIBs, the Fe2O3/SWNT-600 °C composite electrode with the highest Fe2O3 content of 96 wt% delivers an outstanding cycle performance with a stable capacity of 1007.1 mAh g−1 after 100 cycles at 200 mA g−1, as well as a high rate capacity of 567.1 mAh g−1 at 2 A g−1 even up to 600 cycles. These excellent electrochemical properties are attributed to the unique porous and conductive nanoarchitecture accompanied with very high active material loading. In addition, this fabrication method is one-step, tunable, low cost, and can be expanded to the preparation of other transition metal compounds/carbon composites with potential applications in energy and environmental areas such as flexible membrane electrodes for Li-ion and other types of batteries.
Acknowledgments
The authors greatly acknowledge financial support from the National Natural Science Foundation under grants of China NSFC 51502267, Outstanding Young Talent Research Fund of Zhengzhou University (1521317003), Henan province science and technology research project (162102410069), Certificate of Postdoctoral Research Sponsorship Henan Province (2015009). A Cao acknowledges the National Key Research and Development Program of China (Grant No. 2016YFE0127300) and NSFC 51325202.