Abstract
Glyphosate (N-(phosphonomethyl)glycine) is well known nonselective and broad-spectrum herbicide that has been extensively used in agricultural areas around the world to increase agricultural productivity. However, the utilization of glyphosate can cause environmental contamination and health problems. Therefore, the detection of glyphosate with a fast, low-cost, and portable sensor is still important. In this work, the electrochemical sensor has been developed by modifying of working surface on the screen-printed silver electrode (SPAgE) with a mixtures solution between zinc oxide nanoparticles (ZnO-NPs) and poly(diallyldimethylammonium chloride) (PDDA) by the drop-casting process. The ZnO-NPs have been prepared based on a sparking method by using pure zinc wires. The ZnO-NPs/PDDA/SPAgE sensor shows a wide range of glyphosate detection (0 μM–5 mM). The limit of detection of ZnO-NPs/PDDA/SPAgE is 2.84 μM. The ZnO-NPs/PDDA/SPAgE sensor exhibits high selective towards glyphosate with minimal interference from other commonly used herbicides including paraquat, butachlor-propanil and glufosinate-ammonium. Furthermore, the ZnO-NPs/PDDA/SPAgE sensor demonstrates a good estimation of glyphosate concentration in real samples such as green tea, corn juice and mango juice.
Export citation and abstract BibTeX RIS
1. Introduction
Glyphosate (N-(phosphonomethyl)glycine, Gly) is commonly used in global agriculture due to its nonselective, broad-spectrum herbicide and crop desiccant properties. The Gly compound contains three functional groups including carboxylate, amine, and phosphonate which are able to form strong coordination bonds with transition metal ions to give multidentate ligands in a type of bidentate and tridentate complexes [1]. In addition, the commercial herbicide formulations of Gly also improved the efficacy for application in different ways by adding adjuvant additives included in the formulated such as polyoxyethylene amine, POEA, propylene glycol, glycerin, sodium sulfite, sodium benzoate, ascorbic acid, sodium salt of o-phenylphenol, methyl p-hydroxybenzoate, 3-iodo2-propynyl butyl carbamate and 5-chloro-2-methyl 3(2H)-isothiazolone and iso-propylammonium [2, 3]. For this reason, Gly has become one of the most widely used herbicides since its introduction in 1975 (8.6 billion kg). The amount of Gly gradually increased from 51 million kg in 1995 to about 750 million kg in 2014 which caused environmental pollution because the average half-life of Gly has been 32 d depending on the function of microbial activity, soil pH, and temperature [4]. The increasing use of Gly results in residues that are widespread in foods such as fruits and vegetables, and environmental pollutants such as water, soil, and atmosphere which poses health risk like kidney damage, attention deficit hyperactivity disorder (ADHD), diabetes, heart disease, Parkinson's diseases, Alzheimer's and cancer [5–8].
Detection of glyphosate can be done in several techniques such as gas chromatography-Mass Spectrometer (GC–MS), electrochemiluminescence (ELC), UV–vis, Raman spectroscopy, high-performance liquid chromatography (HPLC), photoelectrochemical (PEC), colorimetry, nuclear magnetic resonance (NMR), capillary electrophoresis (CE), and enzyme-linked immunosorbent assays (ELISAs) [9–13]. Although all of these techniques have high sensitivity, they require a complex procedure, costly instrumentations, and are time-consuming. The electrochemical sensor technology has become an alternative efficient method with the advantages of low cost, high sensitivity, low detection limit, high selectivity, simple operation, and rapid analysis [14–16].
Metal oxide nanostructures are attractive materials for various applications due to their unique catalytic, optical, physical, and electronic properties according to their size and shape. Among various metal oxides, zinc oxide (ZnO) is a fascinating material that offers a high diffusion coefficient, high isoelectric point, wide band gap energy (3.37 eV at 300 K), high electron transfer ability and large exciton binding energy (60 meV) at room temperature [17, 18]. Moreover, ZnO nanostructures can be classified into different types of dimensions from 0D to 3D leading to different applications such as photocatalytic activity, solar cells, anti-bacterial activities, supercapacitors, and chemical and biological sensors [19–21]. The ZnO has been synthesized in various methods including hydrothermal synthesis [22], electrochemical deposition [23], chemical vapor deposition [24, 25], spray pyrolysis [26], radio-frequency magnetron sputtering [27], molecular beam epitaxy [28], pulsed laser deposition [29], arc discharge method [30], and sparking method [31].
In this work, we have developed an electrochemical sensor based on the ZnO nanoparticles/poly (diallyldimethylammonium chloride)(ZnO-NPs/PDDA) for glyphosate detection. The PDDA has extensive benefits such as cationic polyelectrolyte, excellent stability, efficient electron transfer ability, good electrocatalytic activity, and remarkable mechanical reinforcement which the strong adhesion of a PDDA layer is capable of bonding between noble metal and metal oxide [32, 33]. The ZnO-NPs were synthesized using zinc wires via the sparking method at room temperature for modification of the working electrode on the flexible screen-printed silver electrode (SPAgE). The choice of the sparking method for synthesizing ZnO-NPs in this study was noteworthy due to its numerous advantages. This method offers a one-step process that is simple, cost-effective, and does not produce any chemical waste. Additionally, it eliminates the need for external temperature and pressure application. Furthermore, the sparking method enables a remarkably rapid synthesis process, as the electrode can be directly deposited within few seconds [19]. For the electrochemical behaviors, they were conducted through cyclic voltammetry (CV), differential pulse voltammetry (DPV), and chronoamperometry (CA) techniques under optimized conditions.
2. Experiments
2.1. Preparation of ZnO-NPs
ZnO-NPs were synthesized by the sparking method in an atmospheric system at room temperature as shown in figure 1. The four pairs of pure zinc wires (purity 99.97%) with a diameter of 0.38 mm were used as anodes and cathodes of sparking tips. First, the experiment started with cleaning the glass substrate by sonication in deionized water, acetone, propanol, and deionized water and dried at room temperature, respectively. Next, the sparking tips of pure zinc wires were set over the 3 ml of deionized water contained in the glass substrate for a distance of 2 mm. The distance between the sparking wires of the anode and cathode was set at 1 mm. Then, a constant voltage of 7.90 V and a current of 3.87 A were set in the sparking machine to form the ZnO-NPs for 3 h. It should be noted that these specific voltage and current values were chosen based on the circuit design of the sparking machine, which incorporated 25 nF capacitor [34]. This configuration was intended to create a strong electric field (internal electric field approximately 10 kV cm−1) capable of sparking the Zn tips (melting temperature of ∼419.5 °C, deposition rate of ∼0.7 nm spark−1, 3 s spark−1 under normal atmosphere) [31]. The 3 h timeframe was selected to ensure the production of a sufficient quantity of ZnO-NPs in water, enabling the deposition of over 100 electrochemical sensors in a single synthesis process. Finally, the ZnO-NPs suspension was loaded in a test tube screw cap for further use at room temperature.
Figure 1. Schematic diagram of sparking method with four pairs of pure zinc wires for formation of ZnO-NPs.
Download figure:
Standard image High-resolution image2.2. Preparation of the ZnO-NPs/PDDA for modifying SPAgE sensor
The modification of the SPAgE sensor with ZnO-NPs is displayed in figure 2. First, 3 ml of ZnO-NPs suspension was sonicated for 30 min. Next, 75 μl of the PDDA solution was added into the ZnO-NPs suspension and further sonicated for 30 min. It should be noted that PDDA acts as the binder between ZnO-NPs and SPAgE to enhance the adhesion property only. Based on CV test, the PDDA did not show any significant peak and effects on glyphosate sensing as demonstrated in figure S1 in the supporting Information. Then, 3 μl of ZnO-NPs/PDDA solution was drop-casted onto the surface of the bare working electrode (Area = 7.065 mm2) three times and dried at room temperature to get the ZnO-NPs/PDDA/SPAgE sensor.
Figure 2. Schematic diagram of the preparation of ZnO-NPs/PDDA for modifying SPAgE sensor.
Download figure:
Standard image High-resolution image2.3. Characterization of ZnO-NPs
The structural morphology of the ZnO-NPs was characterized by high magnification and high-resolution transmission electron microscopy (TEM and HR-TEM, JEOL model: JEM-3100 (HR)). The crystallographic structures of ZnO-NPs were analyzed by x-ray diffraction (XRD, model: Bruker D8 Advance) and a diffractometer with Cu Kα radiation (λ = 1.54 A°) in 2θ range of 20–80°. The chemical and valence states of the ZnO-NPs were examined by x-ray photoelectron spectrometry (XPS, model: Axis Ultra DLD, Kratos analytical, Manchester UK).
2.4. Electrochemical measurements
To investigate the electron transfer reaction mechanism, the CV, DPV, and CA measurements were conducted with a Sensit Smart potentiostat from PalmSens BV. The performance of CV was analyzed for glyphosate by applying a potential range of −0.8 V to +0.8 V and a scan rate of 50 mVs−1 in 10 mM PBS as an electrolyte solution. Data were collected and processed using PSTrace software. All electrochemical measurements were carried out by covering the surface electrode of the sensor with 50 μl of samples and were repeated three times. In addition, a fresh sensor was used to prevent contamination for each measurement.
3. Results and discussion
3.1. Characterization of synthesized ZnO-NPs
Figures 3(a)–(e) shows the formation of ZnO nanoplate due to the agglomeration of ZnO-NPs causing a long time of sparking process. As shown in figure 3(e), the lattice spacing of the crystalline structure of the ZnO-NPs is 0.27 nm which corresponds to the (100) plane of the hexagonal wurtzite ZnO crystal [35, 36]. The EDX elemental mapping (figures 3(b) and (c)) and EDX (figure 3(f)) of the ZnO-NPs structures confirm the main elements and good distribution of Zn and O atoms with the atomic ratio of 36.09:63.91 (Zn:O) for synthesized ZnO-NPs. Therefore, it can be verified that ZnO-NPs were successfully prepared by our proposed sparking method.
Figure 3. (a) the TEM and (b)–(c) their EDS elemental mapping, (d)–(e) the HRTEM and (f) the EDX data of the synthesized ZnO-NPs.
Download figure:
Standard image High-resolution imageThe XRD pattern of ZnO-NPs was recorded in the 2θ range of 20–80° as displayed in figure 4(a). It exhibits peaks positioned at 33.05°, 34.51° and 36.37° corresponding to (100), (002), and (101) ZnO planes (JCPDS card with no. 01-075-1526), respectively. The presence of these peaks confirms a typical hexagonal wurtzite structure of synthesized ZnO-NPs via the sparking method. The crystallite size of ZnO-NPs was calculated to be 27.20–55.60 nm corresponding to the peaks positioned at 33.05°, 34.51° and 36.37° using the Scherrer equation by equation (1). Therefore, the average crystallite size is found to be 39.77 nm. It should be noted that Williamson–Hall (W–H) plot can be also used to identify the crystallite size. However, when considering previous research, it was found that Scherrer's formula provided a more fitted estimation of the crystallite size for the synthesized ZnO-NPs in comparison to the W–H analysis [37]. Therefore, the Scherrer equation was chosen as the preferred method for this study to determine the crystallite size by using equation (1).
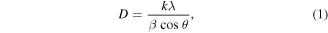
Figure 4. (a) XRD pattern and XPS spectra of (b) survey scan, (c) Zn 2p, and (d) O 1s of the synthesized ZnO-NPs.
Download figure:
Standard image High-resolution imagewhere  is the average crystallite size,
is the average crystallite size,  is the constant value of 0.9,
is the constant value of 0.9,  is the wavelength of
is the wavelength of  line of
line of 
 is the full width at half maximum (FWHM) and
is the full width at half maximum (FWHM) and  is the Bragg's diffraction angle.
is the Bragg's diffraction angle.
To understand the formation mechanism of ZnO-NPs via the sparking method, the high voltage (Eapp) was applied to pure zinc wires. At the end of the zinc wire, the high temperature and pressure will be generated by the bombardment of electrons and ions in a very short time. This phenomenon leads to melting the zinc wire at sparking tips and also oxidizing in the air to form the ZnO. Then, the droplets of ZnO-NPs fall from the sparking tips into the water. A fast change of temperature by water can cause condensation to form the ZnO nanoplate as displayed in the TEM image (figure 3(a)). In addition, the applying energy (Eapp) to metal wires and the synthesis time can affect particle size. Traiwatcharanon et al [38] reported that the size of the synthesized particle was ∼23 nm for a sparking time of 45 min. In this work, the average size of nanoparticles increases significantly to ∼40 nm for a sparking time of 3 h. The effect can be explained by equation (2) [39].
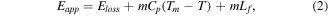
where  is input energy that heats the zinc wires,
is input energy that heats the zinc wires,  is energy loss,
is energy loss,  is the mass of particles,
is the mass of particles,  is heat capacity,
is heat capacity,  is melting point temperature and
is melting point temperature and  is the heat of the fusion of metallic tips.
is the heat of the fusion of metallic tips.
From equation (2) , the right-hand side term is the energy required for bringing the material from room temperature  to the vapor phase. Therefore, the energy was separated into the first term energy loss
to the vapor phase. Therefore, the energy was separated into the first term energy loss  to an environment which was expressed in equation (3) and the second term is the melting energy for zinc wires from room temperature
to an environment which was expressed in equation (3) and the second term is the melting energy for zinc wires from room temperature  to the melting point
to the melting point  and the last is the enthalpy of melting. The mass of particles can be expressed by equation (3).
and the last is the enthalpy of melting. The mass of particles can be expressed by equation (3).

where  is the radius of the spark or the radius of the circular heated spot,
is the radius of the spark or the radius of the circular heated spot,  is spark duration,
is spark duration,  is the mass of particles,
is the mass of particles,  is the thermal conductivity of the zinc wire,
is the thermal conductivity of the zinc wire,  is boiling point temperature and
is boiling point temperature and  is Stefan's constant
is Stefan's constant 
From equation (3), the right-hand side term is heat loss of the heated spot by radiation and thermal conduction, respectively. Part of Stefan's constant will not be considered due to the metal heat radiation emitted less than the black body. The effect of time can be described by the agglomeration state. The nanoparticle can grow bigger with increasing time. At a longer time, the temperature will increase resulting in the size of nanoparticles growing and agglomerating.
To confirm the chemical compositions and valence states of the synthesized ZnO-NPs, x-ray photoelectron spectroscopy (XPS) was used as displayed in figures 4(b)–(d). The XPS survey scan (figure 4(b)) observes the dominant characteristic of Zn and O peaks as major components with no obvious impurity in ZnO-NPs. The devolution of the Zn 2p peak (figure 4(c)) indicates two symmetric peaks at binding energies of 1021 eV and 1044 eV corresponding to the Zn 2p3/2 and 2p1/2, respectively. Based on the binding energy of Zn 2p, the separation peak was 23 eV indicating that the chemical valence of the oxidation state at the surface of the ZnO-NPs was +2 (Zn2+) [40, 41]. The deconvolution of O 1s core level peak (figure 4(d)) which were subdivided into four asymmetric peaks at a binding energy of 530 eV, 531 eV, 532 eV, and 533 eV, respectively. The O 1s state consists of three specific binding energy peaks at low binding energy state peak (LP), middle binding energy peak (MP), and higher binding state peak (HP) corresponding to OLat (I), OVac (II), and OAds (III), respectively [42]. Therefore, the binding energy of 530 eV can be attributed to the lattice oxygen anions  in the oxidation state on the wurtzite structure of ZnO. The 531 eV peak associated with the oxygen vacancies or defects in the lattice
in the oxidation state on the wurtzite structure of ZnO. The 531 eV peak associated with the oxygen vacancies or defects in the lattice  and the 532 eV peak is related to the adsorbed oxygen (chemisorbed oxygen) at the surface [43–50]. It can be observed the initial O 1s peak has FWHM as 2.94 eV after the fit curve it decreases to 1.43 eV for each peak. The possible reason is the chemisorbed oxygen (OCh) on the Zn surface. For the higher binding energy than LP, the MP and HP peaks are associated with oxygen vacancies and surface hydroxide groups. From the XPS results, it is clear that the ZnO-NPs were successfully formed and hydroxide groups may enhance the interaction for the adsorption of glyphosate molecules.
and the 532 eV peak is related to the adsorbed oxygen (chemisorbed oxygen) at the surface [43–50]. It can be observed the initial O 1s peak has FWHM as 2.94 eV after the fit curve it decreases to 1.43 eV for each peak. The possible reason is the chemisorbed oxygen (OCh) on the Zn surface. For the higher binding energy than LP, the MP and HP peaks are associated with oxygen vacancies and surface hydroxide groups. From the XPS results, it is clear that the ZnO-NPs were successfully formed and hydroxide groups may enhance the interaction for the adsorption of glyphosate molecules.
To further investigate the sensing materials on SPAgE electrodes, the XRD patterns of ZnO-NPs/SPAgE and ZnO-NPs/PDDA/SPAgE are shown in figure S2 in the Supporting Information. The ZnO-NPs maintain their wurtzite phase after coating onto SPAgE electrodes. Addition of PDDA binding agent (ZnO-NPs/PDDA/SPAgE) does not exhibit any noticeable peaks that would indicate a significant impact on the phase and structure properties of the main ZnO-NPs sensing material. However, PDDA is still present on SPAgE electrode since N bonding occurs based on XPS analysis as shown in figure S3 in the Supporting Information. It should be emphasized that PDDA has no significant effects on glyphosate sensing (figure S1 in the Supporting Information). Its purpose is solely to improve the bonding between ZnO-NPs and SPAgE. Therefore, ZnO-NPs remain the primary sensing material for glyphosate detection.
3.2. Electrochemical CV analysis of the ZnO-NPs/PDDA/SPAgE sensor
To obtain the high sensitivity of the ZnO-NPs/PDDA/SPAgE sensor for the detection of glyphosate, the parameters including pH of the supporting electrolyte solution (PBS solution) and scan rate should be studied. The peak of oxidation current  reduction current
reduction current  oxidation peak potential
oxidation peak potential  and reduction peak potential
and reduction peak potential  can be directly extracted from CV experiments based on a standard technique [51].
can be directly extracted from CV experiments based on a standard technique [51].
3.2.1. Effect of pH
The electrocatalytic performance of the modified SPAgE sensor with ZnO-NPs was examined by CV using 10 mM of PBS solution containing the glyphosate of 100 μM at a scan rate of 50 mV s−1. The influence of electrolyte solution on the redox (oxidation and reduction) reaction of glyphosate was investigated by varying the pH values of PBS solution from 5.0 to 9.0 as displayed in figure 5(a). As figure 5(b), it can be explained in two main points: firstly, it was observed that a gradual increase in the cathodic peak current  and reached maximum current at pH 6.0. After that, the pH value was continuously increased until pH 9.0 the cathodic peak current began to decrease because the molecules of Gly coordinate with Zn2+ leading to the Gly-Zn2+ complex and the chelating ligands of molecules of amino acids. Secondly, it was observed that the reduction in peak potential
and reached maximum current at pH 6.0. After that, the pH value was continuously increased until pH 9.0 the cathodic peak current began to decrease because the molecules of Gly coordinate with Zn2+ leading to the Gly-Zn2+ complex and the chelating ligands of molecules of amino acids. Secondly, it was observed that the reduction in peak potential  shifts to more negative values with the increase of pH value due to the intervention of protons between Gly and ZnO on the working electrode [52]. The protons can participate in the reduction reaction. Therefore, pH 6.0 was selected to be an optimum parameter for this study.
shifts to more negative values with the increase of pH value due to the intervention of protons between Gly and ZnO on the working electrode [52]. The protons can participate in the reduction reaction. Therefore, pH 6.0 was selected to be an optimum parameter for this study.
Figure 5. CV profiles of a ZnO-NPs/PDDA/SPAgE sensor at (a) different pH values (pH 5, 6, 7, 8 and 9) under scan rate of 50 mV s−1 and 100 μM glyphosate, and (b) corresponding linear calibration plots between pH versus reduction peak currents and potentials.
Download figure:
Standard image High-resolution imageIn addition, the linear regression equations of reduction peak potential  and pH values were found to be:
and pH values were found to be:
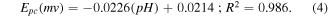
3.2.2. Effect of Scan rate
To describe the kinetics of the ZnO-NPs/PDDA/SPAgE sensor reactions, the CV was recorded at different scan rates over the range from 10 to 50 mV s−1 in PBS solution (pH 6.0) containing 100 μM of glyphosate as displayed in figure 6(a). It can be seen that (I) The peak of oxidation/reduction current  increases as a function of scan rates. (II) The oxidation peak potential
increases as a function of scan rates. (II) The oxidation peak potential  tends to shift to the positive direction value. At the same time, reduction peak potentials
tends to shift to the positive direction value. At the same time, reduction peak potentials  also shift to more the negative direction value. In other words, it can be observed that the peak-to-peak separation
also shift to more the negative direction value. In other words, it can be observed that the peak-to-peak separation  increases (from 39 to 86 mV) with scan rate that indicates a quasi-reversible process and implies slow electron-transfer kinetics in the system.
increases (from 39 to 86 mV) with scan rate that indicates a quasi-reversible process and implies slow electron-transfer kinetics in the system.
Figure 6. CV profiles of a ZnO-NPs/PDDA/SPAgE sensor at (a) different scan rates ranging from 10 mV s−1 to 50 mV s−1 under pH 6.0 and 100 μM glyphosate, (b) plots of the oxidation and reduction peak currents versus scan rate, (c) plot of the square root of scan rates versus reduction currents, and (d) plot of logarithm of scan rates versus peak potentials.
Download figure:
Standard image High-resolution imageThe corresponding plot of the  between scan rates is displayed in figure 6(b). It indicates that both reactions exhibit good linearity with over-increasing scan rates as regression coefficient (R2) 0.971 and 0.990. The linear relation between the cathodic peak current and square roots of scan rates as displayed in figure 6(c), the regression equation, and the correlation coefficient can be expressed as followed
between scan rates is displayed in figure 6(b). It indicates that both reactions exhibit good linearity with over-increasing scan rates as regression coefficient (R2) 0.971 and 0.990. The linear relation between the cathodic peak current and square roots of scan rates as displayed in figure 6(c), the regression equation, and the correlation coefficient can be expressed as followed

This indicates that the electrochemical processes in the ZnO/PDDA/SPAgE sensor occur by the diffusion-controlled process. Moreover, the plot obtained by the plotting between the relationship logarithm of scan rates and cathodic peak current is given by equation (6).
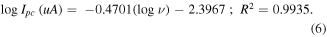
According to the theory, the slope value should be 0.50 which is related to the diffusion-controlled process. From this result, the slope is 0.47 which is close to the theoretical value. In addition, the relation between logarithms at scan rates and  (Tafel plot) is expressed as
(Tafel plot) is expressed as
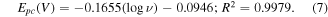
Based on CV experiments with various pH values and scan rates, the reduction potentials of glyphosate are in the range of −0.26 to −0.42 V. It should be noted that the reduction potential of glyphosate is influenced by multiple factors including pH, temperature, scan rate, the choice of sensing material, and the specific electrode employed in the electrochemical measurement. Different studies on glyphosate detection can give the different reduction potentials [53–55].
3.3. Electrochemical DPV analysis of ZnO/PDDA/SPAgE sensor
Based on the evidence of pH and scan rates conditions, the quantitative analysis for the determination of glyphosate on the ZnO-NPs/SPAgE sensor was investigated with a varied concentration level from μM (0–700 μM) to mM (1 –5 mM) using the differential pulse voltammetry (DPV) technique as displayed in figure 7(a). The reduction peak currents of the ZnO-NPs/PDDA/SPAgE sensor decrease continually with increasing glyphosate concentration. This phenomenon may be attributed to the complex formation between glyphosate and Zn2+ resulting in the limiting electron transfer leading to a decrease in the peak current. At low concentrations (0–700 μM), the surface of the ZnO-NPs/PDDA/SPAgE sensor can well absorb glyphosate molecules. Meanwhile, glyphosate molecules were suffocated to absorb on the ZnO-NPs/PDDA/SPAgE sensor at high concentrations (1–5 mM). Therefore, they shows two linear ranges obtained from the current response versus different concentrations of glyphosate as shown in figure 7(b). At lower concentrations (0–700 μM), the regression equation is Ipc (μA) = 0.0082 × CGly(μM) – 14.5670 with R2 = 0.9354. At high concentrations (1–5 mM), the regression equation is Ipc (μA) = 0.00085 × CGly(μM) − 9.4579 with R2 = 0.9962. For the potential, it can be observed that the potential peak of ZnO-NPs/PDDA/SPAgE sensor in the absence of glyphosate (0 μM) is −0.36 V. As the concentration of glyphosate increases, there is a slight shift in the potential, eventually reaching a convergence point at −0.41 V. It is well known that more glyphosate molecules need more energy for reduction process, resulting in causing a progressive shift towards more negative values in the reduction potential at high glyphosate concentration. To compare the reduction potential from CV measurements at the same glyphosate concentration, the reduction potential obtained from the DPV technique is well agreement with the reduction potential conducted using the CV (∼ −0.4 V).
Figure 7. (a) DPV curves and (b) their reduction peak currents of the ZnO-NPs/SPAgE with different concentrations of glyphosate (0 uM to 5 mM). (c) The calibration curve between the changing peak currents and the logarithm of glyphosate concentration.
Download figure:
Standard image High-resolution imageFigure 7(c) shows the calibration curve between the changing peak currents (ΔI = Ino Gly – IGly) and the logarithm of glyphosate concentration. The regression equation is ΔI (μA) = 4.483 Log CGly (μM) − 6.877 with a correlation coefficient of R2 = 0.9916. Moreover, the limit of detection (LOD) efficiency is 2.84 μM, which was determined according to following the equation (8)
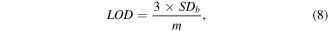
where SDb is the standard deviation of three blank measurements and m is the slope calculated from the calibration curve.
To compare the performance of the ZnO-NPs/PDDA/SPAgE sensor with previous works, the ZnO/PDDA/SPAgE sensor exhibits a wide range detection and low LOD for glyphosate detection as displayed in table 1.
Table 1. Comparison of ZnO-NPs/PDDA/SPAgE sensor with other previously reported sensors for glyphosate detection.
| Sensing materials | Detection method | Linear detection range (μM) | Limit of detection (μM) | References |
|---|---|---|---|---|
| A red-emitting polymerizable guanidinium dyes as fluorescent probes in molecularly imprinted polymers | UV/vis absorption and fluorescence spectroscopy | 0–200 μM | 4.8 μM in CHCl3 | [56] |
| AgNPs | UV–vis extinction spectra SERS | 0–30 μM | 6.0–7.5 μM in water | [57] |
| AgNPs | colorimetric | 5.3 μM | [58] | |
| (UV–vis extinction and surface enhanced Raman spectroscopies) | 18.9 μM | |||
| Polyethylenimine-capped upconversion nanoparticles, copper(II), hydrogen peroxide and 3, 3', 5, 5'-tetramethylbenzidine | Colorimetric | 30–739 μM | 5.91 μM | [59] |
| Agarose-guar gum entrapped bio-nanoconjugate of urease with AuNPs | Potentiometric | 2.96–296 μM | 2.96 μM | [60] |
| CdTe-quantum dots | Fluorometric | 10–118 μM | 3 μM | [61] |
| Fe3O4/molecular-imprinted nanocomposite | Electrochemical | No report | 10 μM | [62] |
| ZnO-NPs/PDDA/SPAgE | Electrochemical | 0–700 μM and 1–5 mM | 2.84 μM | This work |
3.4. Electrochemical CA analysis of ZnO/PDDA/SPAgE sensor
The interference was investigated by a chronoamperometry technique (CA) as displayed in figure 8(a). The CA response was detected by adding other interferences of herbicides (10 mM) like glufosinate-ammonium (Glu-Am), paraquat (PQ), and butachlor-propanil (BP). The performance of the ZnO-NPs/PDDA/SPAgE was studied at a fixed reduction potential applied during measurement  of −0.36 V during the time interval of 360 s. A new interference was added every 60 s in PBS solution (pH 6). After adding the interference as shown in figure 8(b), it was found that the current slightly decreases comparing with the original current of pure glyphosate. The percentage of recovered data (% Recovery) with other interference (glufosinate-ammonium, paraquat, butachlor-propanil) is in the range of 81.57%–95.64%. The results indicate that the ZnO-NPs/PDDA/SPAgE sensor exhibits a high selectivity to glyphosate with less effect on interference of popular herbicides.
of −0.36 V during the time interval of 360 s. A new interference was added every 60 s in PBS solution (pH 6). After adding the interference as shown in figure 8(b), it was found that the current slightly decreases comparing with the original current of pure glyphosate. The percentage of recovered data (% Recovery) with other interference (glufosinate-ammonium, paraquat, butachlor-propanil) is in the range of 81.57%–95.64%. The results indicate that the ZnO-NPs/PDDA/SPAgE sensor exhibits a high selectivity to glyphosate with less effect on interference of popular herbicides.
Figure 8. (a) Chronoamperometric response of the ZnO/PDDA/SPAgE on the addition of different interfering (glufosinate-ammonium, paraquat, butachlor-propanil) and (b) their current responses.
Download figure:
Standard image High-resolution image3.5. Repeatability, reproducibility and stability of the ZnO/PDDA/SPAgE sensor
In order to assess the additional capabilities of the ZnO/PDDA/SPAgE sensors, a set of 30 sensors was manufactured and stored in a closed room at room temperature without controlling the humidity. Among these sensors, 3 sensors were utilized to detect 1 mM glyphosate while the remaining 3 sensors were were dedicated to detecting 5 mM glyphosate using the DPV technique. This experimental process was repeated every 2 days for a duration of 8 days. The average oxidation peak currents along with their corresponding error bars are depicted in figure S4 in the supporting information. Analysis of the results reveals no significant deviation in the current values. The RSDs were found to be below approximately 5%. As a result, these findings confirm the good repeatability, reproducibility, and stability of the ZnO/PDDA/SPAgE sensor.
3.6. Sensing mechanism of glyphosate by the ZnO/PDDA/SPAgE sensor
The possible sensing performance of ZnO-NPs/PDDA/SPAgE sensor for glyphosate is shown in figure 9. The sensing mechanism can be separated into 2 phases as follow;
- Solid phase: at the surface of the electrode, electrons within solid material require higher energy relative to the vacuum level to overcome the surface barrier of the material to create free electrons. When the electrode surface is modified with ZnO-NPs/PDDA material, the Fermi energy level changes to an equal level. Afterward, electrons will transfer from higher to lower Fermi energy levels until the two systems reach to equilibrium and form a new Fermi energy level.
- Liquid phase: in general, glyphosate is a weak acid due to its three dissociation constants at pK1 2.27–2.35, pK2 5.58–5.89, and pK3 10.25–10.89. The solution of glyphosate contains three functional groups such as amines, carboxylates, and phosphonates which behave as donor groups. The pathway of protonation reaction of deprotonated glyphosate starts from one oxygen atom from phosphonates groups, nitrogen atom from the amine group, and oxygen atom from carboxyl, respectively. The phosphonates group is the most reactive to directly bond with the surface of the material.
Figure 9. Possible mechanism of ZnO-NPs/PDDA/SPAgE sensor for glyphosate detection.
Download figure:
Standard image High-resolution imageThe work functions of silver and zinc were 4.7 eV and 5.3 eV, respectively. Contact of the ZnO-NPs/PDDA/Ag will induce electron flow from ZnO to Ag electrode because of the changing of Fermi energy level and formation of new a Fermi energy level. The interactions between ZnO-NPs/PDDA/SPAgE and ligand of glyphosate are controlled by the protonation/deprotonation process. In a solution under the influence of the electrostatic force, the negatively charged donor groups especially phosphonates carboxylates in glyphosate are adsorbed on the surface of positively charged Zn2+. The binding of glyphosate–Zn complexation is expressed in the form of aqueous; ZnL− or ZnHL complexes (L represents the glyphosate ligand) disrupt electron mobility leading to a decrease in the response current. The decrease in current response is relative to the number of glyphosates absorbed onto the surface of the ZnO-NPs/PDDA/SPAgE sensor.
In addition, the dissociation of glyphosate depends on the pH value of the solution. The phosphonates groups can protonate to the ZnO in all pH. In the typical case of pH < pKa1, the COOH group in glyphosate in form (I) dissociates to COO− ion as shown in form (II). For pKa1 < pH < pKa2, the glyphosate molecules in form (I) disappeare and transform to form (II) and (III). In case of form (III), it was found that the proton-donor (P-OH) dissociates into the proton-acceptor (P-O−) and there is still one proton left which is protonated to nitrogen (NH2+). The interaction of the carboxylate group with the ZnO surface is discovered to be a weak reaction. Above the pH > pKa3, the positive charge (the amino group) has remained protonation but the phosphonates and carboxylate groups will completely be dissociated and precipitated.
3.7. Real sample analysis of ZnO/PDDA/SPAgE sensor
Considering the variation in permissible levels of glyphosate contaminants in drinking water across countries [63, 64], a concentration of 4 μM of glyphosate was intentionally added to real samples including green tea, corn juice and mango juice. These real samples were purchased from a supermarket in Thailand. The DPV technique was employed to measure the reduction peak currents of ZnO-NPs/PDDA/SPAgE sensors upon the real sample with 4 μM glyphosate contamination. The resulting currents were then utilized in a linear calibration curve (figure 7(b)) to determine the concentration of glyphosate. The analytical results are summarized in table 2. The percentage recoveries of glyphosate detection in the real samples vary from 103.8% to 126.8% with a % RSD between 3.5% and 5.1%. These values indicate a reliable estimation of glyphosate concentration in the real samples. However, in the case of corn juice, there appears to be a slight overestimation of the glyphosate concentration. This discrepancy could be attributed to additional ingredients such as creamer or milk in instant corn juice, potentially causing a higher percentage recovery compared to other real samples.
4. Conclusion
In conclusion, the ZnO-NPs/PDDA/SPAgE has been successfully developed for glyphosate analysis with the electrochemical techniques. The ZnO-NPs can be achieved by sparking of pure zinc wires via the sparking method that offers a simple, rapid, low-cost process and no chemical waste. The crystallite size of the synthesized ZnO-NPs was 27.20–55.60 nm. For fabrication of electrochemical sensor, the screen-printed electrode was modified with the ZnO-NPs on the working electrode surface to act as a sensing material. The electrochemical behaviors have been investigated using CV, DPV, and CA. To reach the high sensitivity of the ZnO-NPs/PDDA/SPAgE sensor, the pH 6 and scan rate of 50 mV s−1 were found to be the optimal parameter conditions. The ZnO-NPs/PDDA/SPAgE sensor presented two linear responses with concentrations of glyphosate at a level of μM (0–700 μM) and mM (1–5 mM). The ZnO-NPs/PDDA/SPAgE sensor was able to detect glyphosate concentrations as low as 2.84 μM. The % recovery of interferences was in the range of 81.57%–95.64% indicating to a high selectivity to glyphosate with less effect on interference of popular herbicides including glufosinate-ammonium, paraquat, and butachlor-propanil. Additionally, this electrochemical sensor based on ZnO-NPs/PDDA/SPAgE demonstrates accurate estimation of glyphosate levels in various real samples including green tea, corn juice, and mango juice.
Acknowledgments
This work was financially supported by the Office of the Ministry of Higher Education, Science, Research and Innovation; and the Thailand Science Research and Innovation through the Kasetsart University Reinventing University Program 2021 and Kasetsart University Research and Development Institute (FF(KU) 25.64).
Data availability statement
All data that support the findings of this study are included within the article (and any supplementary files).
Declaration of competing interest
The authors declare no competing interest.
Supplementary data (1.1 MB DOCX)










