Abstract
Click chemistry is not a single specific reaction, but describes ways of generating products which emulate examples in nature. Click reactions occur in one pot, are not disturbed by water, generate minimal and inoffensive byproducts, and are characterized by a high thermodynamic driving force, driving the reaction quickly and irreversibly towards a high yield of a single reaction product. As a result, over the past 15 years it has become a very useful bio-orthogonal method for the preparation of chemical cross-linked biopolymer-based hydrogel, in the presence of e.g. growth factors and live cells, or in-vivo. Biopolymers are renewable and non-toxic, providing a myriad of potential backbone toolboxes for hydrogel design. The goal of this review is to summarize recent advances in the development of click chemistry-based biopolymeric hydrogels, and their applications in regenerative medicine. In particular, various click chemistry approaches, including copper-catalyzed azide-alkyne cycloaddition reactions, copper-free click reactions (e.g. the Diels–Alder reactions, the strain-promoted azide-alkyne cycloaddition reactions, the radical mediated thiol-ene reactions, and the oxime-forming reactions), and pseudo-click reactions (e.g. the thiol-Michael addition reactions and the Schiff base reactions) are highlighted in the first section. In addition, numerous biopolymers, including proteins (e.g. collagen, gelatin, silk, and mucin), polysaccharides (e.g. hyaluronic acid, alginate, dextran, and chitosan) and polynucleotides (e.g. deoxyribonucleic acid), are discussed. Finally, we discuss biopolymeric hydrogels, cross-linked by click chemistry, intended for the regeneration of skin, bone, spinal cord, cartilage, and cornea. This article provides new insights for readers in terms of the design of regenerative medicine, and the use of biopolymeric hydrogels based on click chemistry reactions.
Export citation and abstract BibTeX RIS
1. Introduction
Tissue engineering-based regenerative medicine is a promising biomedical field, where 'intelligent' biomaterials, together with biological factors, including growth factors and stem cells, are used to promote the healing process of damaged tissues or organs. Biomaterials not only provide tissue template scaffolds for cell proliferation and migration, but also act as platforms to spatially localize and control the release growth factors. Therefore, the design and fabrication of 'intelligent' biomaterials is a key component of tissue regeneration. One major class of instructive biomaterials are hydrogels, i.e., three-dimensional (3D) water-swollen polymeric networks containing large amounts of water, typically 70%–90% [1, 2]. Hydrogels may provide a microenvironment with similarities to the extracellular matrix (ECM), which is characterized by a highly hydrated porous yet interconnected structure, permitting nutrient and metabolite exchange. The requirements for hydrogel biomaterials in regenerative medicine often include: (1) injectability, facilitating the minimally-invasive delivery of material to the injury site, (2) the controlled release of drugs, growth factors or cells at the injury site, (3) comparable mechanical properties with surrounding tissues, (4) biocompatibility, and (5) biodegradability to non-toxic products, enabling easy elimination from the body. In recent years, hydrogels have been recognized as excellent candidates for various regenerative medical applications, such as wound healing [3–5], bone regeneration [6, 7], spinal cord regeneration [8], cartilage regeneration [9, 10], and regeneration of the cornea [11, 12].
The polymer backbones of hydrogels may be derived from synthetic polymers, for example, Pluronic®F127 [13], polyethylene glycol (PEG) [14], polyvinylpyrrolidinone [15] or from biopolymers classified as proteins (e.g. collagen, gelatin, silk fibroin (SF) and mucin), polysaccharides (e.g. hyaluronic acid (HA), alginate, dextran and chitosan) and polynucleotides (e.g. deoxyribonucleic acid (DNA)). Compared with synthetic polymers, biopolymers have received greater attention, due to their biocompatibility, bioactivity and biodegradability. The cross-linking methods for hydrogels include the formation of chemical bonds, providing for physical interaction, and the combination of physical and chemical networks. Physical interactions include hydrophobic interaction, hydrogen bond cross-linking, van der Waals forces, ionic interaction, and crystal cross-linking. Compared with physical cross-linkages, chemical bonds-based networks of the same polymer typically demonstrate greater stability and gel stiffness, and slower degradation rates, providing more sustained spatial supports for a slow regeneration process. Common chemical cross-linking methods for hydrogel preparation include the free radical polymerization approach, and click chemistry reactions. However, UV light illumination, horseradish peroxidase (HRP), or other radical initiators are required for the free radical cross-linking method, with the concomitant risk of potential biological toxicity for living cells and tissues. Conversely, click reactions are defined as chemical reactions which are rapid, spontaneous, versatile, extremely selective, and result in high yields of products when two molecular substances or components are mixed or react together, even under mild reaction conditions [16]. Hydrogels cross-linked by click reaction have grown in popularity, owing to their robust and selective reactivity in physiological environments [17, 18]. This chemical strategy allows for a variety of synthetic strategies to accomplish the cross-linking and chemical functionalizing of hydrogels with tailored physical, chemical, and biological properties. Hubbell and team performed pioneering work to develop Michael addition hydrogels for tissue regeneration in the 1990's [19]. Thereafter, many scientists have explored new click reactions to develop hydrogels for biomedical applications.
In this review, we focus on biopolymeric hydrogels, cross-linked by click reactions, and their applications for regenerative medicine. In section 2, we introduce different click reactions that have been used to cross-link polymer networks. In section 3, we introduce various biopolymers used to prepare hydrogels, providing detailed information in terms of polymer selection. Finally, we discuss medical applications for these hydrogels. This review provides new insights into the design, preparation, and regenerative medical applications of biopolymeric cross-linked hydrogel materials.
2. Click reactions
Click chemistry is a very useful bio-orthogonal method for preparing chemical cross-linked hydrogel. In this section, we summarize the various click reactions (table 1) that have been used to cross-link polymer networks, including (1) copper-catalyzed azide-alkyne cycloaddition (Cu-AAC) reactions; (2) copper-free click reactions (Diels–Alder (DA) reactions, Strain-promoted azide-alkyne cycloaddition (SPAAC) reactions, radical mediated thiol-ene reactions, and oxime-forming reactions); (3) pseudo-click reactions, including thiol-Michael addition reactions and Schiff base reactions.
Table 1. List of click chemistry reactions employed to cross-link hydrogels.
| Type of click reaction | Cross-linked chemical structure | Catalyst | Advantages | Disadvantages | Reference |
|---|---|---|---|---|---|
| Cu-AAC reaction |
| Cu(I) | Simplicity; high yield; high efficacy; no by-production | Cytotoxicity of Cu(I) ions | [20, 21] |
| |||||
| DA reaction |
| No | High versatility; high selectivity | Longer reaction time; soft resulting gel | [22–25] |
| |||||
| SPAAC reaction |
| No | No toxic copper catalyst; biocompatibility | Hydrophobicity of the cyclooctane disturbs cross-linking reaction; synthesis of the cyclooctane and difluorinated cyclooctynes are difficult | [26, 27] |
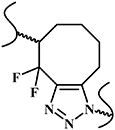
| |||||
| Radical mediated thiol-ene reaction |
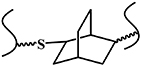
| UV/Vis | High reaction rates, high yield; high selectivity; mild reaction conditions; biocompatibility | UV light is harmful to cells | [28, 29] |
| Oxime-forming reaction |
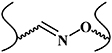
| No | Fast reaction kinetics; water as the only side product; mild reaction condition | Precursor may be challenging to synthesize. | [30–32] |
| |||||
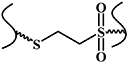
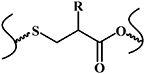
| Thiol-Michael reaction |
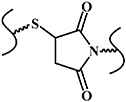
| No | Mild reactive conditions; controllable reaction rate; high chemical yield | Thiols of protein interfere with the reaction | [12, 33, 34] |
| |||||
| Schiff base reaction |
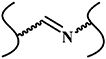
| No | Fast reaction kinetics; no toxic by-product; high reaction efficiency | Cross-linkage of reversibility result into unstable hydrogel | [5, 13, 25, 32, 35–43] |
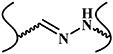
|
2.1. Copper-catalyzed azide-alkyne cycloaddition (Cu-AAC)
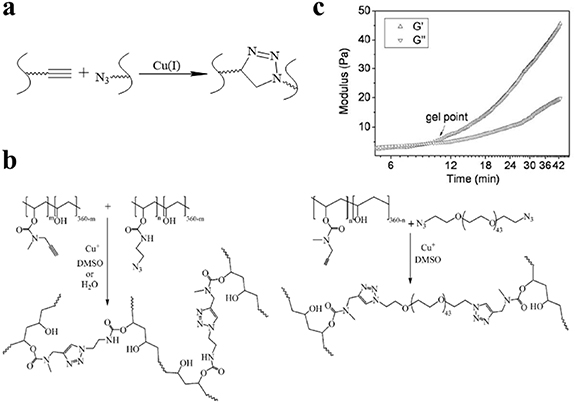
Cu-AAC, the azide-alkyne Huisgen cycloaddition, is a copper-catalyzed 1,3-dipolar cycloaddition between an alkyne to give a 1,2,3-triazole (figure 1(a)) [44], which is the most representative approach in click chemistry. The Cu-AAC reaction has been demonstrated as a highly effective approach to preparing in situ hydrogel, owing to its simplicity, high yield, high efficacy, and lack of by-products [45, 46]. To prepare the hydrogel precursors, the azide or alkyne is linked to the polymer chain by modifying relevant functional groups [47, 48]. Our group pioneered the use of the Cu-AAC reaction to design hydrogel networks, where an azide-modified poly(vinyl alcohol) (PVA) was cross-linked with telechelic bifunctional PEG-diazide or alkyne-modified PVA in a water solution, together with a Cu(I) catalyst (figure 1(b)) [20]. Zhang et al used the same cross-linking reaction to prepare thermosensitive hydrogels formed between azide-modified cellulose and alkyne-modified poly(N-isopropylacrylamide-co-hydroxylethyl methacrylate), in which the sol-to-gel transition point occurred within 10 min after mixing (figure 1(c)) [21].
Figure 1. Click hydrogel preparation via the Cu-AAC reaction. (a) Schematic diagram of Cu-AAC reaction. (b) Cross-linking reaction between alkyne-modified PVA and azide-modified PVA or PEG-diazide, leading to gel formation; Reprinted from [20], copyright (2006), with permission from the American Chemical Society. (c) Oscillatory rheology of hydrogels formed between azide-modified cellulose and alkyne-modified poly(N-isopropylacrylamide-co-hydroxylethyl methacrylate); Reprinted from [21], copyright (2009), with permission from Elsevier.
Download figure:
Standard image High-resolution image2.2. Copper-free click reaction
Due to the toxicity of the copper catalyst [49] and the easy oxidization of Cu(I) ions in water [50], such hydrogels have not proved useful for tissue regeneration. Copper-free click reactions have therefore been developed to replace Cu-AAC. Copper-free click reactions include the DA reactions, the SPAAC reactions, the radical mediated thiol-ene reactions, and oxime-forming reactions.
2.2.1. Diels–Alder (DA) reaction.
DA reactions (figure 2(a)) always occur between diene and olefin (or dienophile) without catalysts or coupling agents [16], and are characterized by their high reaction rate and selectivity [16, 49, 51]. For DA-based systems, maleimide-furan reactions are the most frequently used reaction to prepare hydrogels for tissue regeneration (figure 2(a)). For example, Nimmo et al developed HA-based hydrogels using furan-modified HA and bis-maleimido PEG in a slightly acidic environment, where the mechanical properties and degradation properties of the hydrogel could be adjusted by controlling the molar ratio of furan to maleimide (figure 2(b)) [22]. In addition, Guaresti et al described a stimuli-responsive chitosan-based hydrogel using furan-modified chitosan and a bismaleimide cross-linker (figure 2(c)) [24]. As expected, the swelling degree of the chitosan hydrogel was strongly dependent on the quantity of bismaleimide group of polymer backbone in the acidic medium, where the increased cross-linkage decreased the swelling ratio by 25% [24].
Figure 2. Click hydrogel cross-linked via the DA reaction. (a) Schematic diagram of DA reaction; (b) Schematic representation of the formation of HA-based hydrogels by cross-linking HA-Furan with bis-maleimido PEG via DA reaction; Reprinted from [22], copyright (2011), with permission from the American Chemical Society. (c) Chitosan hydrogel formation through DA reaction; Reprinted from [24], copyright (2018), with permission from Elsevier.
Download figure:
Standard image High-resolution image2.2.2. Strain-promoted azide-alkyne cycloaddition (SPAAC) reaction.
The SPAAC reaction is an aromatic triazole-formed reaction between a cyclooctyne and an azide moiety (figure 3(a)), and can occur without a catalyst, even at room temperature [16, 52]. Fu et al reported injectable HA-PEG hydrogels, cross-linked via the SPAAC click reaction under physiological conditions, where the prepared hydrogel exhibited a short time to gelation, good strength, slow degradation, and outstanding cell-compatibility (figure 3(b)) [26]. Truong et al used the SPAAC reaction to fabricate a photo-degradable hydrogel, based on dibenzylcyclooctyne-C4-acid-modified PEG and azide-modified gelatin macromolecules (figure 3(c)) [27]. Two weeks of 3D cellular culture results indicated the hydrogel with good cytocompatibility [27]. Moreover, encapsulated fibroblasts could also be released on-demand, due to the cleavage of the nitrobenzyl moiety after UV light irradiation; the released cells displayed no differences in comparison with the parent fibroblasts [27].
Figure 3. Click hydrogels cross-linked by the SPAAC reaction. (a) Schematic diagram of SPAAC reaction. (b) Preparation of HA-PEG hydrogel using azide-PEG and cyclooctyne-HA via SPAAC reaction. Reprinted from [26], copyright (2017), with permission from Elsevier. (c) Reaction scheme for SPAAC chemistry, and representative scheme for the formation of PEG-Gelatin hydrogels and UV induced degradation. Reprinted from [27], copyright (2015), with permission from the American Chemical Society.
Download figure:
Standard image High-resolution image2.2.3. Radical mediated thiol-ene reaction.
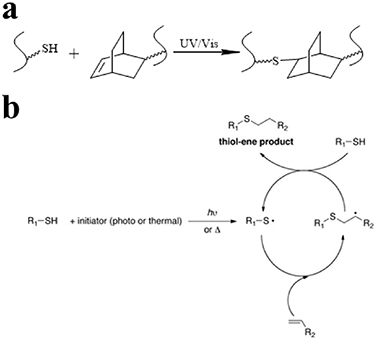
The radical mediated thiol-ene reaction is a click reaction between a thiol group and an alkene moiety of the reactants to form a thioether, initiated either by light illumination or by a thermal initiator (figure 4(a)) [53, 54]. The thiyl radicals are induced from the thiol group via the initiator, which can react with the carbon-carbon double ('-ene') bond to form a carbon-sulfur bond (figure 4(b)). Liang et al recently reported a composite hydrogel using poly(ethylene glycol) diacrylate (PEGDA), and silk fibroin (SF), cross-linked using a 405 nm UV illumination mediated thiol-ene click reaction, in which the cross-linkage was formed from the acrylate moiety on PEG backbones and glutathione on SF [28]. The cross-linked PEGDA/SF hydrogel demonstrated the effective sustained-release behaviors of rhodamine B, combined with a limited swelling ratio, as well as excellent biocompatibility in vivo [28]. Lueckgen et al developed UV-initiated photo-cross-linked alginate hydrogels using norbornene-modified alginate, a photoinitiator, and two different thiol-coupled peptides (RGD peptide and degradable cross-linker), resulting in a cell-empowered enzymatic degradable material [29].
Figure 4. (a) Schematic diagram of radical mediated thiol-ene reaction. (b) Reaction mechanism of radical mediated thiol-ene reaction. Reprinted from [53], copyright (2016), with permission from Wiley.
Download figure:
Standard image High-resolution image2.2.4. Oxime-forming reaction.
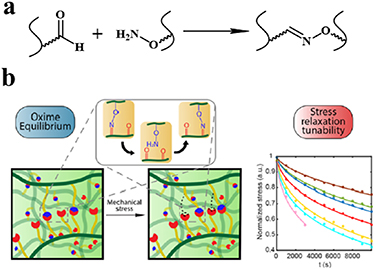
The oxime-forming reaction takes place between an amino-oxy functionality and an aldehyde or ketone (figure 5(a)). This reaction is high rate, orthogonal, and non-catalyzed, and its byproduct is water [55]. Moreover, the stability of oximes can be fine-tuned by virtue of the reversibility of the linkage. Oxime-forming reactions have been demonstrated as an approach to preparing hydrogel biomaterials with slow degradation rates, using more stable oximes [56]. Saénchez-Moraén et al introduced alkoxyamine and aldehyde groups to an alginate polymer backbone [57]. The oxime-forming reaction between alkoxyamine groups and aldehyde groups of alginate was then used to prepare alginate hydrogel at 37 °C (figure 5(b)) [57]. The hydrogel exhibited an adjustable stress relaxation behavior, and manifested effective biocompatibility in a subsequent in vitro Mus musculus B lymphoma cells encapsulated experiment [57]. Hardy et al reported an in situ cross-linking injectable hydrogel, comprising aldehyde HA and aminooxy-terminated PEG [31]. The mechanical and swelling properties of the injectable hydrogels were tunable, based on the composition of the hydrogels, to values analogous with soft tissues [31].
Figure 5. Hydrogels cross-linked by the oxime-forming reaction. (a) Schematic diagram of oxime-forming reaction. (b) Schematic description of oxime cross-linked alginate hydrogel network and its reorganization on the application of external mechanical stress or strain, and stress relaxation profiles. Reprinted from [57], copyright (2019), with permission from the American Chemical Society.
Download figure:
Standard image High-resolution image2.3. Pseudo-click reaction
With increased in-depth research into click chemistry, a class of reactions known as pseudo-click reactions have attracted the attention of the biomaterials community: these primarily include thiol-Michael addition reactions and Schiff base reactions [16, 17].
2.3.1. Thiol-Michael addition reaction.
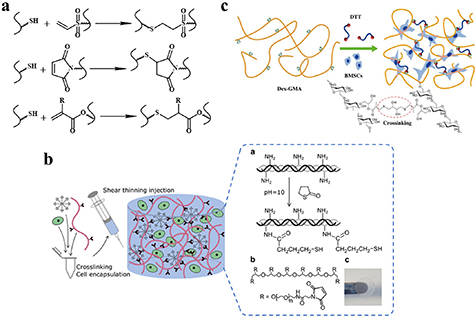
The thiol-Michael addition reaction occurs between thiol and vinyl groups (figure 6(a)) [58], and can be initiated by a wide variety of catalysts, such as bases, metals, organometals, Lewis acids, and nucleophiles. Of these catalysts, bases and nucleophiles are the most efficient, with the least propensity for side reactions. Although both base- and nucleophile-catalyzed thiol-Michael reactions proceed rapidly, with high conversion rates under mild conditions, their mechanisms are slightly different. In the traditional base-catalyzed thiol Michael addition reaction pathway, the base abstracts a proton from the thiol to generate a thiolate anion, which subsequently undergoes the thiol-Michael addition reaction, whereas in the nucleophile-mediated pathway, the nucleophile reacts with the electron-deficient double bond to generate an intermediate carbanion, which, in turn, deprotonates the thiol to generate a thiolate anion. Recently, our group reported an injectable shape-holding collagen hydrogel based on thiol group-modified collagen and 8-arm PEG with maleimide moieties [33], which demonstrated shear-thinning, self-healing properties, and low swelling ratio (6%). The viability of mesenchymal stem cells (MSCs) and human umbilical vein endothelial cells (HUVEC) encapsulated in the resulting hydrogel were more than 80% and 70%, respectively, after a 4 day 3D culture period, indicating the presence of sufficient nutrient and oxygen diffusion in the hydrogel culture system (figure 6(b)) [33]. Liu et al reported an in situ thiol-Michael hydrogel using glycidyl methacrylate derivatized dextran backbones, cross-linked by dithiothreitol, under physiological conditions [34]. The mechanical properties, gelation time, and swelling behavior of the hydrogel was strongly dependent on a system of pH values. Moreover, the results of 3D cell encapsulation experiments revealed high viability levels for MSCs and NIH/3T3 fibroblasts (figure 6(c)) [34].
Figure 6. Hydrogels cross-linked via thiol-Michael addition reactions. (a) Schematic diagram of thiol-Michael addition reaction; (b) Schematic for thiol-Michael collagen hydrogel formation. Reprinted from [33], copyright (2019), with permission from the American Chemical Society. (c) Schematic for dextran hydrogel formation after DTT addition. Reprinted from [34], copyright (2015), with permission from Elsevier.
Download figure:
Standard image High-resolution image2.3.2. Schiff base reaction.
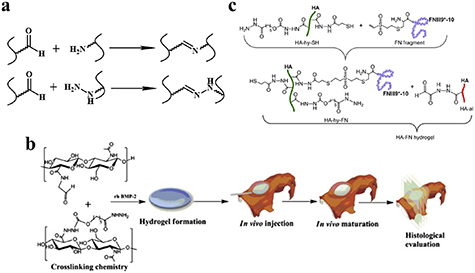
The Schiff base reaction is one of the most popular methods for developing biocompatible hydrogels. The reaction occurs between amino (R-NH2) or hydrazide (R-NHNH2) and aldehyde (R-CHO) functional groups, forming imine or acylhydrazone with acid-responsive covalence (figure 7(a)) [59, 60]. Chen's group synthesized a novel biocompatible polysaccharide-based self-healing hydrogel, comprising N-carboxyethyl chitosan, adipic acid dihydrazide, and oxidized sodium alginate [32]. The results of rheological recovery testing, macroscopic observation, and beam-shaped strain compression measurement revealed excellent self-healing ability with a high healing efficiency (≈95%) [32]. Moreover, NIH3T3 fibroblast cells were encapsulated during the gel formation, maintaining a high viability and proliferative capacity [32]. Yin et al developed mussel-inspired injectable hydrogels, featuring hydrazide-modified poly(L-glutamic acid) and dual-functionalized alginate (catechol- and aldehyde-modified alginate). The hydrogels exhibited effective self-healing capability, good cytocompatibility and strong bio-adhesion [35]. Our group developed several hydrazine cross-linked in situ injectable hydrogel systems, based on HA macromolecules, for different biomedical applications, such as tissue regeneration [61–63], biomineralization [64], and magnetic resonance imaging [65, 66]. Here, the HA was modified with aldehydes and hydrazides, respectively. On mixing these two components, the hydrogel formed within 30 s at physiological pH (figure 7(b)) [61]. This hydrogel was subsequently used to deliver bone morphogenetic protein-2, and was found to promote bone regeneration in animal experiments, indicating its potential orthopedic application [61]. Moreover, the Schiff base HA hydrogel can not only act as a growth factor delivery platform, but can also support MSC attachment and spreading, when conjoined with integrin-specific fibronectin fragments [62] (figure 7(c)).
Figure 7. Hydrogels cross-linked via the Schiff base reaction. (a) Schematic diagram of Schiff base reaction. (b) HA hydrogel as an rhBMP-2 carrier for in vivo bone augmentation using a minimally invasive technique. Reprinted from [61], copyright (2011), with permission from Elsevier. (c) The process of hydrogel preparation and conjunction with integrin-specific fibronectin fragment. Reprinted from [62], copyright (2013), with permission from Elsevier.
Download figure:
Standard image High-resolution image3. Selecting biopolymers
Biopolymers are produced in nature by living organisms, and are classified into three main types: proteins, polysaccharides, and polynucleotides. Those polymers found in the human body are degraded by the body, producing enzymes to be eliminated by native pathways as required. In this section, we introduce those biopolymers most frequently employed in cross-linking hydrogel via click reactions. In particular, protein-based polymers (e.g. collagen, gelatin, silk fibroin (SF) and mucin), polysaccharides (e.g. hyaluronic acid (HA), alginate, dextran, and chitosan (CS)), as well as polynucleotides (e.g. DNA) are highlighted (see table 2), providing some new ideas for readers in terms of the selection of appropriate polymer backbones.
Table 2. List of click chemistry cross-linked biopolymers for regenerative purposes.
| Type | Biopolymers | Schematic diagram | Types of click chemistry | Demonstrated regenerative medicine application |
|---|---|---|---|---|
| Protein | Collagen | / | SPAAC [67]; Oxime-forming reaction [31]; Schiff base reaction [42, 43, 68–70]; | Wound healing [42, 43]; Cornea regeneration [71] |
| Gelatin | / | Cu-AAC reaction [72, 73]; DA reaction [74–76]; SPAAC reaction [27]; radical mediated thiol-ene reaction [77–80]; Schiff base reaction [38]; thiol-Michael reaction [12] | Wound healing [36]; Bone regeneration [38]; Spinal cord regeneration [8]; Cornea regeneration [12, 71] | |
| Silk protein |
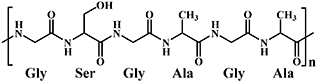
| Radical mediated thiol-ene reaction [81]; Schiff base reaction [82, 83]; | / | |
| Mucin | / | DA reaction [84] | / | |
| Polysaccharides | Hyaluronic acid |
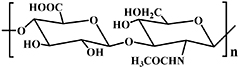
| Cu-AAC reaction [72]; Schiff base reaction [37, 39–41, 85]; Radical mediated thiol-ene reaction [86]; DA reaction [76, 87]; Oxime-forming reaction [31]; thiol-Michael reaction [85, 88] | Wound healing [37]; Spinal cord regeneration [39–41]; Cartilage regeneration [88]; Cornea regeneration [11] |
| Alginate |
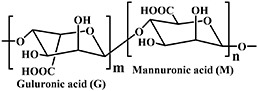
| Schiff base reaction [5, 25, 89, 90]; Oxime-forming reaction [57]; Radical mediated thiol-ene reaction [29]; DA reaction [25, 91] | Wound healing [5]; Bone regeneration [25, 38]; Cartilage regeneration [92]; | |
| Dextran |
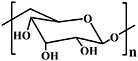
| Thiol-Michael reaction [93]; Schiff base reaction [5, 36, 94]; | Wound healing [5, 36]; | |
| Chitosan |
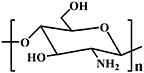
| DA reaction [95]; Schiff base reaction [13, 96, 97] | Wound healing [13]; Cartilage regeneration [97] | |
| Polynucleotides | DNA | / | aza-Michael addition [98] | / |
3.1. Protein
3.1.1. Collagen.
Collagen is the most abundant protein in mammalian tissue, constituting approximately 30% of all proteins, and providing structural and mechanical support for the body [99–102]. Collagen can be divided into three main types: type I, occurring in skin, tendon, and bone tissue; type II, existing in cartilage tissue; type III, existing in skin and vascular tissue [102]. In general, collagen extracted from mature tissue is highly cross-linked and insoluble; however, water-soluble and acid-soluble collagen can be obtained from young tissue [102]. Collagen comprises three polypeptide α-chains, wound together in a tight triple helix fiber (see figure 8(a)). Each individual polypeptide chain is composed of approximately 1000 amino acid residues. In terms of the composition of the amino acids, collagen is characterized by glycine (33%), proline (12%–14%), 4-hydroxyproline (<14%) and 4-hydroxylysine (15%). Collagen, as a well-established hydrogel raw material, exhibits biocompatibility, biodegradability, and does not produce any harmful residues, increasing its attractiveness for tissue engineering and regenerative medicine applications [103].
Figure 8. (a) Collagen triple helix structure; Note: HYP = Hydroxyproline; GLY = Glycine; PRO = Proline; Reprinted from [104], copyright (2016), with permission from Wiley. (b) Collagen modified with DBCO-sulfo-NHS and azido-PEG5-NHS; modified collagens were then mixed and cross-linked via bio-orthogonal SPAAC under physiological conditions. Reprinted from [67], copyright (2020), with permission from American Chemical Society.
Download figure:
Standard image High-resolution imageSPAAC, oxime-forming and Schiff base reactions are the most common methods of cross-linking collagen networks in a water phase. For example, Chen et al modified collagen with azide and dibenzocyclooctyne moieties, respectively, via the NHS coupling reaction. The collagen hydrogels were cross-linked via the SPAAC reaction, simply mixing the two modified collagen variants at 1:1 (v/v) ratio under physiological conditions (figure 8(b)) [67]. The Schiff base reaction is another attractive method of cross-linking collagen networks. Zhang et al manufactured hydrogels using aldehyde dextran, and collagen via the Schiff base reaction. The resulting hydrogels exhibited improved mechanical properties, achieving a maximum compressive strength of up to 32.5 ± 1.6 kPa, around 20 times higher than that of a pure collagen hydrogel [69].
3.1.2. Gelatin.
Gelatin is the product of multi-stage hydrolysis of type I collagen, and a heterogeneous mixture of water-soluble proteins of high average molecular weights. Depending on its preparation method, gelatin can be classified as Type A Gelatin (where the raw materials are pretreated in an acidic medium, after which the gelatin is extracted in an acidic medium) and Type B Gelatin (where the raw materials are pretreated in alkaline medium, and the gelatin is then extracted in a neutral medium) [105, 106]. Gelatin is temperature-responsive, with water solubility above ambient temperature, and insolubility at low temperature. Furthermore, cell adhesion, differentiation, and proliferation are promoted by its binding peptide (arginine-glycine-aspartic acid peptide (RGD)) [105, 107]. Moreover, it exhibits sensitivity for endogenous matrix metalloproteinase, facilitating biodegradability in vivo [108].
Physical cross-linked gelatin hydrogel is spontaneously induced by hydrogen bonds in and between the molecular chains [109]. However, the low stiffness of physical cross-linked gelatin hydrogels limits their application in regenerative medicine. Consequently, click chemical cross-linking methods including Cu-AAC reaction [72, 73], DA reaction [74–76], and radical mediated thiol-ene reaction [77–80], have been employed to enhance the stiffness of gelatin hydrogels. For example, Hu et al introduced alkynyl groups into gelatin, using propiolic acid to modify it [72]. The propiolic acid-modified gelatin, azido-modified HA and azido-modified chitosan were then employed to prepare hydrogels via the Cu-AAC reaction [72]. The resulting hydrogel exhibited the characteristics of an elastic hydrogel, and promoted cell proliferation and adhesion, while demonstrating good cytocompatibility [72]. Yu et al synthesized furancarboxylic acid-modified gelatin by introducing furan to gelatin [76]. A DA reaction between the furan group modified gelatin and maleimide of PEG was employed to prepare a biomimic hydrogel in an MES buffer (pH 5.5) [76]. The mechanical properties and degradation rate of the gelatin hydrogel could be readily adjusted by changing the molar ratio of the furan and the maleimide [76]. Moreover, gelatin hydrogels can also be formed by photo-mediated radical mediated thiol-ene reactions, where cross-linking occurs between thiolated gelatin and norbornene/methacrylamide-functionalized gelatin [80]. The resulting hydrogel could be used as bioink, with excellent biocompatibility and printability, for applications in the field of additive manufacturing [80].
3.1.3. Silk fibroin (SF).
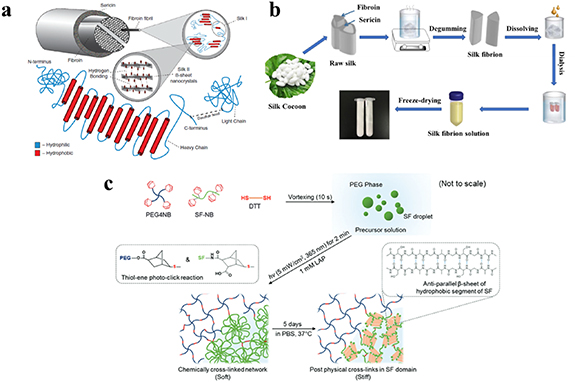
Silk Fibroin (SF) is extracted from silk cocoons containing 18 different amino acids, in which glycine, alanine and serine account for more than 80% of the total composition [110, 111]. The raw silk is mainly composed of sericin and SF, where sericin, a soluble glycoprotein, is located on the outermost layer of silk, covering the SF (figure 9(a)) [112]. The SF is constructed of fibroin fibrils, whose conformation can be divided into two categories: silk I, consisting of random coil and α-helix, and silk II, a β-sheet [113, 114]. Fibroin fibrils are composed of heavy (approximately 325–350 kDa) and light (approximately 25 kDa) chains, connected by a disulfide bond [112]. The amino acid sequences of the heavy and light chains are different. The heavy chain is mainly composed of hydrophobic and hydrophilic regions, connecting alternately [112]. SF possesses properties favorable for biomedical applications, and is widely used in regenerative medicine [115–117]. The extraction process of SF, shown in figure 9(b), is well established. Firstly, the silk is cut into small pieces, and is then degummed by boiling in a sodium carbonate solution to obtain the SF. The degummed SF is dissolved in a solution of 9 M lithium bromide or 50% (WT) calcium chloride under heating conditions to transform the hydrophobic β-sheet into a hydrophilic α-helix conformation. Finally, the dissolved silk fibroin solution is dialyzed and lyophilized to obtain regenerated SF which is soluble in water [118].
Figure 9. (a) The structure of silk; Reprinted from [112], copyright (2018), with permission from Wiley. (b) Preparation process for soluble silk fibroin; Reprinted from [118], copyright (2020), with permission from Elsevier. (c) Preparation schematics for silk fibroin-PEG hydrogel, cross-linked via a radical mediated thiol-ene reaction system; Reprinted from [81], copyright (2016), with permission from the Royal Society of Chemistry.
Download figure:
Standard image High-resolution imageRyu et al introduced the norbornene group into SF backbones at pH 9.5, using a borate buffer (figure 9(c)) [81]. Thereafter, click SF microgel was fabricated via a radical mediated thiol-ene reaction with dithiothreitol (DTT) [81]. Interestingly, the storage modulus of the bulk hydrogel could be further increased by means of a five day incubation in PBS, inducing stiff β-sheet formation [81]. Chen et al prepared a hydrogel by combining SF with oxidized dopamine via the Schiff base reaction and self-polymerization of free oxidized dopamine at pH 8.5 [83]. The physicochemical property of the hydrogel was easily adjustable by means of altering the concentration of the components of the hydrogel. Hippocampal neurons cultured in this gel showed the longest axon length when the hydrogel was prepared using 2 mg ml−1 dopamine [83].
3.1.4. Mucins.
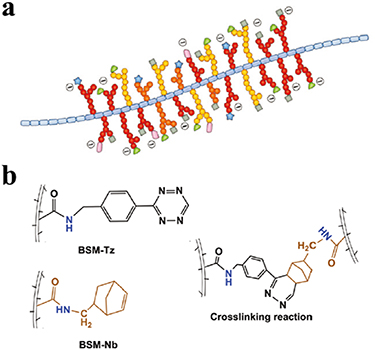
Mucins are glycoproteins with a molecular weight greater than 200 kDa. All types of mucin molecules possess a protein in their core which is decorated with a number of glycosylated regions. The sugar chains are primarily connected to the protein core by O-type glycosidic bonds (figure 10(a)) [119]. Mucin occurs prolifically in living creatures, and can be extracted from animals such as snails, jellyfish, pigs, and cows, as well as from eggs [119]. The main function of mucin is to prevent our mucous membranes from becoming dehydrated, and to avoid damage from mechanical pressure when swallowing food [119]. Furthermore, Mucin is a physical barrier, serving to prevent the ingress of harmful molecules and viruses, which is the first line of defense in the protection of epithelial cells [119]. Mucin not only protects cells, but also acts as a biologically active molecule to regulate cell function. With the aim of preventing immune-mediated foreign body response to hydrogel implantation in the body, Yan et al used tetrazine (Tz) and norbornene (Nb) conjugated mucin to prepare a hydrogel via the DA reaction (figure 10(b)) [84]. Experimental results indicated that the hydrogel did not elicit fibrosis when implanted in the abdominal cavity of C57Bl/6 mice for a period of 21 d [84].
Figure 10. (a) Common structural features of mucin molecules; Reprinted from [119], copyright (2018), with permission from the Royal Society of Chemistry. (b) Preparation schematics for mucin hydrogel, cross-linked via DA reaction system; Reprinted from [84], copyright (2019), with permission from Wiley.
Download figure:
Standard image High-resolution image3.2. Polysaccharides
3.2.1. Hyaluronic acid.
Hyaluronic acid (HA), the only non-sulfated glycosaminoglycan (GAG), is one of the most abundant polysaccharides, occurring in many mammalian tissues and body fluids, including connective tissue, skin, synovial fluid, vitreum, and dental pulp matrix [120]. HA is composed of the repeating disaccharide units of β-D-glucuronic acid and N-acetyl-D-glucosamine, alternately linked by β-1,3 and β-1,4 glycosidic bonds (see table 2) [121]. Depending on its molecular weight, HA can be divided into five categories: <10 kDa of oligosaccharides of HA; 10–250 kDa of low molecular weight HA; 250–1000 kDa of medium molecular weight HA; >1000 kDa of high molecular weight HA; and >6 × 103 kDa of super high molecular weight [122]. In the past, HA was sourced from comb extraction; however, the HA used in today's market is mainly derived from bacterial expression systems in Streptococci. As a major component of ECM, HA demonstrates many in vivo biological functions, such as tissue lubrication, moisture holding, angiogenesis, and tissue regeneration [123–125].
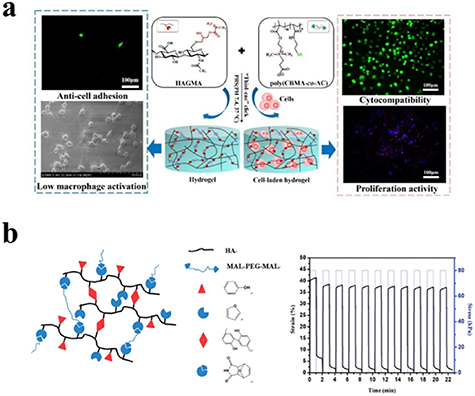
Endogenously present hyaluronidase (HAase) can degrade HA hydrogel chains in vivo by breaking the 1,4-linkages between 2-acetamido-2-deoxy-b-D-glucose and D-glucose [126]. HA-based hydrogels have been widely used for biomedical applications such as cell carriers, growth factor releasing platforms, regenerative scaffolds, etc. Several click chemistry methods have been employed to prepare these gels. For example, our group prepared Schiff base and thiol-Michael addition reaction cross-linked HA hydrogels, respectively, to load a bioactive growth factor (BMP-2) [85]. Interestingly, both in vivo and in vitro experiments demonstrated that the BMP-2 in the Schiff base-based hydrogel displayed higher bioactivity than that of the Michael addition-based hydrogel [85]. Zhang et al prepared a non-fouling and cytocompatible HA hydrogel using methacrylated HA with a thiol-functionalized zwitterionic carboxybetaine methacrylate copolymer via a radical mediated thiol-ene click reaction (figure 11(a)) [86]. The resulting HA hydrogel exhibited biodegradability, self-healing, and resistance to protein absorption and cell adhesion (figure 11(a)) [86]. Yu et al integrated simultaneously enzymatic cross-linking and DA click chemistry to prepare an injectable HA hydrogel that demonstrated superior shape memory and resistance to fatigue [87]. In addition, its storage modulus and breakage strength were nearly 27 kPa and 109.4 kPa, respectively. The hydrogel was able to resist 80 kPa for 1 min, even after 10 cycles of loading-unloading measurement, and the corresponding deformation was completely recovered within 1 min on withdrawal of the loading force (figure 11(b)) [87].
Figure 11. (a) Hydrogel cross-linked via radical mediated thiol-ene reaction between methacrylated HA and thiol-functionalized zwitterionic carboxybetaine methacrylate copolymer for 3D cell encapsulation and culture in vitro. Reprinted from [86], copyright (2020), with permission from Elsevier. (b) Schematic diagram of formation of HA hydrogel, and the hydrogel in DMA creep mode with 80 kPa loading stress for 10 cycles. Reprinted from [87], copyright (2014), with permission from the Royal Society of Chemistry.
Download figure:
Standard image High-resolution image3.2.2. Alginate.
Alginate is a naturally occurring linear block anionic polysaccharide, with an average molecular weight of about 240 kDa. Alginate proliferates in the cell walls of brown algae, combining with various cations in seawater to form a range of alginates (see figure 12(a)). The extract obtained from brown algae is generally sodium alginate. Alginate can also be synthesized by different bacteria, such as Pseudomonas and Azotobacter, via microbial fermentation [127, 128]. The structure of alginate is composed of homopolymerized α-l-guluronate (G block), homopolymerized β-d-mannuronate (M block), and alternating blocks of the two uronic acids above, connected by 1,4-glycosidic bonds. It can be characterized as mannuronic acid (M block) and guluronic acid (G block) units, arranged in an irregular block-wise pattern of varying proportions of GG, MG, and MM blocks [128–131]. The monomer composition ratio and sequence of G and M have a significant impact on the functional properties of the alginate.
Figure 12. (a) Schematic of the chemical structure of alginate; (b) Reaction schematic illustration of composite hydrogel integrated with GMs containing TH via the Schiff base reaction; Reprinted from [89] Copyright (2016), with permission from Elsevier.
Download figure:
Standard image High-resolution imageAlginate has gained much attention in terms of scientific reasearch and industrial applications, owing to its structural and chemical resemblance to ECM, as well as desirable characteristics such as biocompatibility, high moisture absorption, and relatively low cost [128, 132]. Chen et al fabricated a composite hydrogel using oxidized alginate (OAlg), carboxymethyl chitosan (CMCS), and tetracycline hydrochloride (TH) loaded gelatin microspheres (GMs) in PBS via the Schiff base reaction (figure 12(b)) [89]. The resulting hydrogel exhibited improved mechanical properties and antibacterial activity, by virtue of the introduction of tetracycline hydrochloride-loaded gelatin microspheres [89]. Lueckgen et al modified alginate via the coupling of norbornene groups using carbodiimide chemistry. The alginate hydrogel was cross-linked via a UV-initiated thiol-ene reaction after mixing the norbornene-modified alginate, photoinitiator, and peptide cross-linkers in PBS [29]. The results showed that the degradation rate of the hydrogel could be controlled and adjusted by cross-linker type and collagenase concentration [29]. The alginate hydrogels were then employed to encapsulate mouse embryonic fibroblasts, which remained viable in these hydrogels, indicating their capacity to provide a suitable microenvironment for cells [29]. Wang et al synthesized furan-modified alginate via an amidation reaction [91]. Thereafter, a DA reaction and a radical mediated thiol-ene reaction between the furan-modified alginate, dimaleimide poly(ethylene glycol), and HHC10-CYS antimicrobial peptide were utilized to prepare antimicrobial hydrogels at 37 °C [91]. The results demonstrated that the hydrogels synthesized in this study possessed excellent antibacterial properties, in achieving an antimicrobial rate of 100% with the addition of an antimicrobial peptide [91]. Alginate, however, is not found in vertebrates, and therefore lacks the native enzymes to facilitate degradation in vivo. Furthermore, unlike endogenous ECM components, alginate does not have specific biological functions.
3.2.3. Dextran.
Dextran is secreted by bacteria, and is non-toxic, neutral, and hydrophilic; it consists of linear α-(1→6) linked D-glucose units (see table 2) [133], representative of a type of polysaccharide with glucose unit molecules connecting with glycosidic bonds. Dextran is the first commercially available microbial polysaccharide, and is produced by Leuconostoc mesenteroides and S. mutans bacteria. Based on its specific glycosidic bonds, dextran can be divided into α-dextran and β-dextran. The structure and molecular weight of dextran differs based on the types and fermentation conditions of the microorganisms utilized. Several click chemistry reactions have been used to prepare dextran-based hydrogels.
Nonsuwan et al oxidized glycidyl methacrylate dextran in order to introduce aldehyde groups to glycidyl methacrylate dextran using sodium periodate [93]. The dextran-based hydrogel was prepared using aldehyde glycidyl methacrylate dextran solution with DTT via the thiol-Michael click reaction [93]. The degradation rate of the resulting hydrogel could be enhanced by increasing the addition amount of the amino source in the incubation medium, in which the amino source reacted with aldehyde groups on the dextran backbones to cleave the polymer chain [93]. Du et al constructed an injectable hydrogel, using oxidized dextran and dodecyl aldehyde-modified chitosan via the Schiff base reaction; the resulting hydrogel exhibited multifunctional properties, such as superior hemostasis, antibacterial activity, tissue adhesion, and cytocompatibility [94]. The hydrogel demonstrated effective antibacterial properties against S. aureus and P. aeruginosa, respectively [94]. It is significant to note that dextrane does not readily degrade in vivo, but that soluble dextrane can be slowly metabolized by the liver [134].
3.2.4. Chitosan (CS).
CS is the one of most abundant polysaccharides in nature. It is a linear copolymer, composed of repeated units of D-glucosamine and N-acetyl-D-glucosamine (see table 2) [133, 135, 136]. The main source of the CS precursor, chitin, is the shells of shrimp and other crustaceans [133]. Alkaline deacetylation and enzymatic degradation methods are often used to obtain CS from chitin [133]. Due to the hydrogen bond between the hydroxyl group and the amine group, CS exhibits poor solubility under neutral or alkaline conditions [133]. Furthermore, the solubility of CS depends on the degree of deacetylation and the molecular weight of CS, as well as the ionic strength of the solution [133].
Compared with other biopolymers, CS not only demonstrates biocompatibility and biodegradability, but also possesses antibacterial qualities [135]. Several click chemistry reactions, such as the DA reaction and the Schiff base reaction, have been employed to prepare CS-based hydrogels. For example, Guaresti et al manufactured a biocompatible CS-based hydrogel using furan-functionalized CS and maleimide-functionalized CS via the DA reaction [95]. The prepared hydrogel showed a certain pH-sensitivity, excellent antibacterial activity, and good biocompatibility [95]. Zhao et al prepared quaternized CS-g-polyaniline, which exhibited superior antibacterial properties and water solubility [96]. An injectable hydrogel with self-healing properties was fabricated using a Schiff base reaction cross-linkage between quaternized CS-g-polyaniline and benzaldehyde group functionalized poly(ethylene glycol)-co-poly(glycerol sebacate) [96]. The resulting hydrogel showed free radical scavenging capability, electrical activity, adhesion, electrical conductivity, antibacterial activity, and in vivo biocompatibility [96]. Similarly to alginate and dextrane, chitosan is not naturally occurring in vertebrates, and lacks the specific enzymes for degradation. However, it has been shown to degrade on exposure to lysozyme [137].
3.3. Polynucleotides
Polynucleotides are a class of biopolymer, which is the general term for deoxyribonucleic acid (DNA) and ribonucleic acid (RNA). Polynucleotides represent biological macromolecular compounds, polymerized by many nucleotide monomers, and are among the most basic substances in biological molecules, occuring in all animal and plant cells and microorganisms. The nucleotide is the basic unit of polynucleotides, comprising 5-carbon sugars, phosphate groups, and nitrogen-containing bases. If the 5-carbon sugar is ribose, the polymer formed is RNA, in which the nitrogenous bases primarily include adenine (A), guanine (G), cytosine (C), and uracil (U). If the 5-carbon sugar is deoxyribose, the polymer formed is DNA, in which the nitrogenous bases mainly include adenine (A), guanine (G), cytosine (C), and thymine (T). In the double helix structure of DNA, the bases on one chain must be paired with the bases on the other chain, based on purine and pyrimidine base pairing rules (figure 13(a)).
Figure 13. (a) DNA double helix structure. (b) Aza-Michael addition-based cross-linking of biomass DNA using PEGDA and biomass DNA. (c) Biomass DNA hydrogels made from blue-green algae, E. coli, onion, and salmon testes. (Top) Photographs of DNA hydrogels; (bottom) fluorescence images of DNA hydrogels stained by DNA specific dyes: GelRed or SYBR Green I. Reprinted from [98] Copyright (2020), with permission from American Chemical Society.
Download figure:
Standard image High-resolution imageIn recent years, DNA, as a biopolymer, has begun to be used to prepare hydrogels whose specific advantages include stability, precise controllability, facile synthesis and modification, and flexibility [138]. In 2016, Li et al reviewed the application of DNA hydrogels in biomedicine [138]. The two main types of DNA hydrogels are DNA-hybrid hydrogel and pure DNA hydrogel [138]. The team also discussed the research status of DNA hydrogels in recent years [138]. Recently, Wang et al used PEGDA and biomass DNA from blue-green algae, E. coli, onion, and salmon testes, respectively, to prepare DNA hydrogels via aza-Michael addition (see figures 13(b) and (c)) [98]. The tensile stress and Young's moduli of the DNA hydrogels were shown to gradually increase with increasing DNA content [98]. The DNA hydrogel displayed good cell compatibility in relation to Caco-2 and MCF-7 cells when the DNA hydrogel concentration in the culture media was around 13.1% (w/v) [98].
4. Click biopolymeric hydrogels for regenerative medicine
The hydrogel-mediated tissue engineering approach has been widely applied in the field of tissue and organ regeneration. In this section, we review the various applications of click chemistry-based biopolymeric hydrogels in skin, bone, spinal cord, cartilage, and cornea regenerative treatments (figure 14).
Figure 14. Regenerative medicine applications based on biopolymeric hydrogels, cross-linked by click chemistry.
Download figure:
Standard image High-resolution image4.1. Wound healing
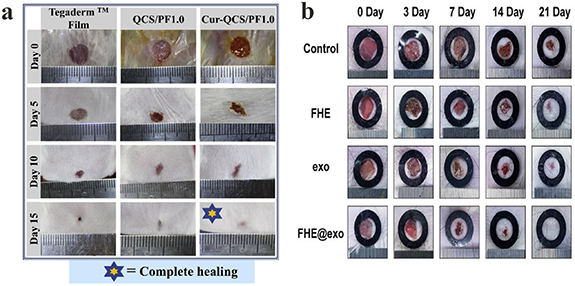
Skin, as the largest organ in the human body, plays a crucial role in protecting the body from infection, irradiation, high temperature, etc. Skin damage is normally induced by burning, scratching, or wounding. Minor skin defects may heal spontaneously, whereas serious wounds such as full-skin burns require treatment to accelerate healing. The wound healing process generally comprises four typical stages: hemostasis, inflammation, proliferation, and remodeling [136, 139], and involves the interaction between different types of cells, bioactive factors, and ECM compounds [140]. The process of wound healing begins with hemostasis and inflammation. These two stages involve the recruitment of platelets and immune cells to control blood loss and eliminate pathogens [141]. The initially recruited immune cells play a major role in secreting chemokines and growth factors, which attract cells and guide the healing process to the next stage of proliferation [130]. The proliferation phase includes a series of processes, such as the development of granulation tissue (the formation of a temporary extracellular matrix), vascularization, and re-epithelialization (the formation of an epidermal skin layer), leading to contraction of the wound's surface. This special stage is regulated by a variety of cells, primarily fibroblasts, endothelial cells, macrophages, and keratinocytes. The final stage is the remodeling process, in which the previously formed matrix undergoes a gradual transformations into functional skin or scar tissue [141, 142]. To accelerate this four-stage healing process, many click chemistry-based biopolymeric hydrogels, with and without drugs/growth factors, have been tested on various defect areas. Hu et al prepared a series of new aminoglycoside hydrogels with acid responsiveness [5]. The antibacterial hydrogels were prepared using oxidized polysaccharides (such as dextran, carboxymethyl cellulose, sodium alginate and chondroitin) and cross-linking agents, via the Schiff base reaction [5]. The experimental results indicated that the hydrogels possessed outstanding antibacterial properties, both in vitro and in vivo [5]. Qu et al reported a series of multifunctional injectable hydrogels with adhesion, hemostasis, self-healing, stretching, and inherent antibacterial properties, as a new type of joint skin wound dressing [13]. The hydrogel was prepared using a quaternized chitosan solution, was cross-linked with PF127-aldehydes via the Schiff base reaction [13]. These hydrogel dressings demonstrated superior promotion of the healing process, as compared to commercial (Tegaderm™) dressings, enahancing granulation tissue growth and collagen synthesis, and up-regulating the expression of vascular endothelial growth factors in a full-thickness skin defect model (figure 15(a)) [13]. Chen et al developed a self-healing hydrogel-encapsulated chlorhexidine acetate and basic fibroblast growth factor for wound healing [36]. This facilitated the rapid delivery of the chlorhexidine acetate, so as to inhibit inflammation at the early stages of wound repair, and the sustainable release of the basic fibroblast growth factor to stimulate cell proliferation and wound repair at the wound site [36]. The results indicated that the hydrogel promoted wound healing by reducing inflammation, promoting collagen fiber growth and fibroblast proliferation [36]. Recently, Wang et al prepared a multifunctional hydrogel dressing (FHE hydrogel) cross-linked via the Schiff base reaction, using Pluronic F127 (F127), oxidized HA and  -polylysine (EPL) [37]. This hydrogel had several functions, such as rapid hemostasis, self-healing, tissue adhesion, high-efficiency antibacterial activity, and resistance to multiple drug-resistant bacteria [37]. Adipose-derived MSC-derived exosomes were loaded into the hydrogel via electrostatic interaction, and the hydrogel exhibited a sustained pH-responsive release behavior [37]. In vivo studies in rats showed that the hydrogel dressing loaded with exosomes could stimulate the angiogenesis process of wound tissue to promote wound healing, and could also promote cell proliferation, granulation tissue formation, collagen deposition, remodeling and re-epithelialization, leading to faster healing for serious wounds (figure 15(b)) [37].
-polylysine (EPL) [37]. This hydrogel had several functions, such as rapid hemostasis, self-healing, tissue adhesion, high-efficiency antibacterial activity, and resistance to multiple drug-resistant bacteria [37]. Adipose-derived MSC-derived exosomes were loaded into the hydrogel via electrostatic interaction, and the hydrogel exhibited a sustained pH-responsive release behavior [37]. In vivo studies in rats showed that the hydrogel dressing loaded with exosomes could stimulate the angiogenesis process of wound tissue to promote wound healing, and could also promote cell proliferation, granulation tissue formation, collagen deposition, remodeling and re-epithelialization, leading to faster healing for serious wounds (figure 15(b)) [37].
Figure 15. (a) Photographs of wounds at 0th, 5th, 10th and 15th day after applying QCS/PF1.0 hydrogel and Cur-QCS/PF1.0 hydrogel. Reprinted from [13] Copyright (2018), with permission from Elsevier. (b) Representative images of wound healing process treated with FHE, exosomes, FHE@exo. Reprinted from [37] Copyright (2019), with permission from Ivyspring International Publisher.
Download figure:
Standard image High-resolution image4.2. Bone regeneration
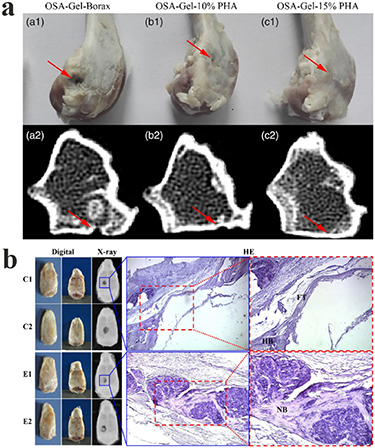
The function of bone, is to protect of internal organs, maintain posture, and transfer power to move the body; it is mainly composed of a natural complex of collagen and hydroxyapatite. Bone has a multilayered structure, and can be divided into cancellous and cortical bone. Cortical bone is located on the outer layer of bone, having a high weight bearing capacity, and its center is a blood vessel surrounded by bone matrix [143]. Bone has the capacity to regenerate by itself by means of a sophisticated process [144], which includes osteoinduction and regeneration, involving many types of cells and factors. Serious injuries or diseases, however, such as trauma, infection, arthritis, etc, do not regenerate, and therefore require new supplementary therapies. In recent years, the emergence of hydrogels, particularly click chemistry-based biopolymeric hydrogels, has seen a proliferation of research publications in relation to bone regeneration applications [131, 145]. Liu et al used the Schiff base reaction to prepare a mussel-inspired bone repair hydrogel under mild conditions, between dopamine decorated nanohydroxyapatite (PHA), oxidized sodium alginate (OSA) and gelatin (Gel) [38]. The experimental results revealed that the gelation time of the hydrogel was 3 to 7 min, providing sufficient injection time for clinical settings [38]. In vitro studies revealed that the hydrogel promoted bone marrow mesenchymal stem cells (BMSCs) cell adhesion, proliferation, and osteogenic differentiation [38]. The results of the CT images and histological evaluation of their in vivo studies demonstrated that a hydrogel with PHA could accelerate the regeneration of bone (figure 16(a)) [38]. In another interesting study, Bai et al used adipic dihydrazide-modified sodium alginate, PEG-AMI, the thermosensitive copolymer, F127@ChS, and bio-glass to prepare a triple cross-linked hydrogel via the Schiff base reaction, the DA reaction, and noncovalent cross-linking, for the purpose of bone regeneration [25]. The triple cross-linked hydrogel with bio-glass showed increased stiffness, with a storage modulus higher than the triple cross-linked hydrogel without bio-glass [25]. The results of x-ray images showed that bone-like tissue appeared in the experimental group [25]. According to HE histopathological analysis, only fibrous tissue and a small amount of bone tissue appeared in the defect site of the control group; interestingly, mature/mineralized bone tissue could be seen in the defect site of the experimental group (figure 16(b)) [25].
Figure 16. (a) Photograph and CT images of bone defect injected with hydrogels after 12 weeks. Reprinted from [38] Copyright (2019), with permission from Wiley. (b) Bone repair evaluation after a 12-week regeneration period, using digital images, x-ray imaging and HE histopathological analysis. C and E represent control (treated with PBS) and experimental (treated with hydrogel with bio-glass) groups, respectively. HB: host bone; FT: fibrous tissue; NB: new bone. Reprinted from [25] Copyright (2017), with permission from Elsevier.
Download figure:
Standard image High-resolution image4.3. Spinal cord regeneration
The spinal cord is an important part of the central nervous system. The main function of the spinal cord is to transmit sensory and motor information between the brain and the peripheral nerves. Spinal cord is also a low-level center for simple reflexes, such as urination and defecation. Spinal cord injury (SCI) is a type of severe central nerve injury, causing partial or complete loss of sensory and motor function below the injury site [146]. In addition to neurological dysfunction, patients with SCI often experience various complications, including pressure sores, urinary complications, respiratory complications, deep vein thrombosis and pulmonary embolism, spasm, pain, vegetative hyperreflexia, and osteoporosis [147]. These complications not only affect the process and effectiveness of rehabilitation therapy, but also seriously affect the patient's quality of life, and can in some cases be life-threatening. There are approximately 13.3 ~ 22.6 ten thousand new cases of SCI each year, worldwide. Expensive treatment costs, long-term rehabilitation treatment, and the loss of employment have a huge impact on individuals and families [148]. The regeneration of the spinal cord is therefore very significant but equally challenging. Hydrogel-assisted tissue engineering is a potential method to facilitate SCI healing.
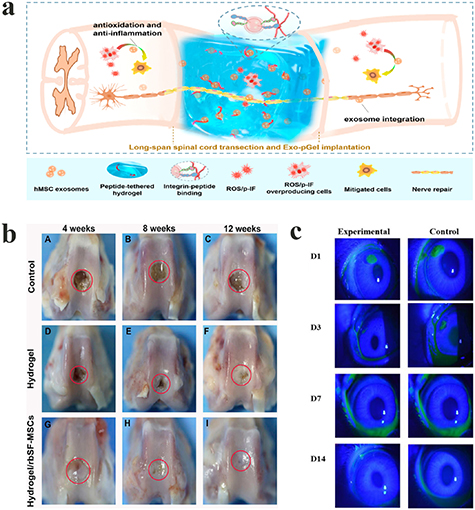
Stem cells have the capacity to self-renew, and can differentiate into functional cells of specific tissues, which can then be used as seed cells in various tissue regeneration and repair processes. A variety of cells, including MSCs, neural stem cells, embryonic stem cells, olfactory ensheathing cells, oligodendrocytes, etc are used for transplantation to repair spinal cord injury. Li et al used a cross-linked HA hydrogel, together with a binding PPFLMLLKGSTR peptide to encapsulate MSCs and MnO2 nanoparticles. The results of animal experiments showed that the transplantation of MSCs helped the differentiation of nerve cells in SCI, promoting the recovery of motor function of SCI rats [39]. In addition to motor function deficits, trauma at the SCI injury site can cause a series of secondary pathophysiological reactions, including ischemia and hypoxia, the infiltration of inflammatory cells, and the release of excitatory amino acids and free radicals, resulting in the breakdown of axons and myelin sheaths, and even the death of nerve cells. Therefore, it is useful for SCI healing to implant hydrogel materials into the SCI site for the purpose of immunomodulation. For example, Zhang et al synthesized an antioxidant hydrogel via the Schiff base reaction, cross-linking between aldehyde HA, adipic acid dihydrazide graft HA and 2,2,6,6-tetramethylpiperidinyloxy [40]. In the peroxidized microenvironment simulated in vitro, the antioxidant hydrogel exhibited obvious antioxidant effects [40]. Following implantation in the body, the hydrogel was shown to significantly promote the recovery of motor function in rats with spinal cord transection [40]. Moreover, exosomes derived from the MSCs were encapsulated into peptide-HA hydrogel, together with binding PPFLMLLKGSTR [41], in which exosomes could sustainably be released in the injured spinal cord tissue, thereby decreasing inflammation and the peroxidation microenvironment, and promoting the recovery of motor function in SCI rats (figure 17(a)) [41].
Figure 17. (a) Illustration of therapeutic use of HA-based hydrogel loaded with exosomes for SCI. Reprinted from [41] Copyright (2020), with permission from the American Chemical Society. (b) Photographs of rabbit knee articular defects at 4, 8 and 12 weeks after hydrogel/rbSF-MSCs injection. The red circles indicate the original defect margin. Reprinted from [97] Copyright (2019), with permission from Elsevier. (c) Representative images of corneal wound area stained with sodium fluorescein on day 1, day 3, day 7, and day 14. Reprinted from [12] Copyright (2018), with permission from the American Chemical Society.
Download figure:
Standard image High-resolution image4.4. Cartilage regeneration
Cartilage is found in human synovial joints, as well as the spine, ribs, external ear, nose, respiratory tract, and growth plates in children and adolescents. Human cartilage is mainly divided into three types: hyaline cartilage, fibrous cartilage, and elastic cartilage. Hyaline cartilage is widely distributed, and has a typical structure [149]. Histologically, the cartilage can be divided into four structural regions, with different compositions and arrangement. The thin superficial area has a large number of chondrocytes, which stretch into an oblong shape and are parallel to the joint surface. This area is characterized by high cell density and collagen fibers parallel to the joint surface. The cell density in the adjacent transition zone is low, and the collagen fibers and cells are arranged randomly. The cell density in the deep zone is the lowest, and the collagen fibers are perpendicular to the tissue surface [150, 151].
There are more than 150 million people with damaged articular cartilage in the world, according to the estimates of the World Health Organization [152]. Adult cartilage tissue has no blood vessels and nerves, limiting the ability of cartilage tissue to repair itself after injury. Several animal experiments have been conducted using click chemistry-based biopolymeric hydrogels for the regeneration and repair of cartilage, where the use of stem cells in conjunction with the hydrogel material, represents the most common treatment approach. For example, Yao et al prepared a HA-based hydrogel, using thiolated HA and maleimided HA via the thiol-Michael reaction [88]. The results of in vivo experimentation showed that the HA-based hydrogel loading bioactive collagen type I could effectively promote cartilage repair by improving chondrocyte adhesion and proliferation, and enhancing the genetic expression level associated with hyaline cartilage formation [88]. Jia et al prepared a chitosan-based hydrogel, using benzaldehyde-capped poly (ethylene oxide) and glycol chitosan via the Schiff base reaction [97]. Rabbit synovial fluid mesenchymal stem cells (rbSF-MSCs) were then encapsulated in the chitosan-based hydrogel [97]. Assessment of the in vivo repair indicated that the hydrogel/rbSF-MSCs facilitated cartilage repair in relation to MSC chondrocyte differentiation and proliferation (figure 17(b)) [97].
4.5. Cornea regeneration
The cornea is a transparent thin-film tissue covering the front surface of the eyeball, containing one-sixth of the thickness of the extraocular fiber membrane [153], which plays a mechanical role in protecting the internal structure of the eyeball, and acts as physical barrier to prevent external harmful substances from entering the eye [154, 155]. In addition, the cornea allows light to pass through the pupil and converge into the fundus of the retina for imaging, similarly to the lens of a camera [156]. The cornea is easily damaged, since it is in the outermost layer of the eye tissue [153]. There are many reasons for corneal damage, including eye trauma (mechanical and chemical), bacterial infection, burns, or viral infections.
Corneal injury repair comprises a series of complex physiological processes, involving corneal cells, epithelial cells, stromal cells, and endothelial cells, as well as growth factors and the remodeling of ECM [153]. It is therefore very difficult to regenerate the cornea. With the continuous exploration and development of tissue engineering in the field of regenerative medicine, researchers are gradually working to find or develop new corneal replacement and regenerative repair materials. Corneal regeneration materials must have a similar water content, light transmittance and ion transmission rate to the natural cornea, and require a certain mechanical strength to withstand surgical sutures and post-operative care, but also possess a certain stability (resistant enzymatic hydrolysis capability) and good biocompatibility to support corneal epithelial cell growth. Hydrogels possess all of the required properties for promoting corneal regeneration, and have become the major focus of current research [157].
Li et al manufactured photocurable hydrogels, using acrylated and thiolated gelatin as a repair material for the regeneration of local corneal wounds [12]. Animal experiments demonstrated that the hydrogel had good biocompatibility in vivo with rabbit corneas, and facilitated the repair of local corneal wounds, exhibiting epithelial wound coverage within 3 d (figure 17(c)) [12]. Moreover, Koivusalo et al developed tissue adhesive HA-DOPA hydrogels by grafting dopamine moieties into hydrazine cross-linked hyaluronic acid for corneal regeneration [11]. The human adipose-derived stem cells (hASCs) encapsulated in HA-DOPA hydrogels exhibited good proliferation and cell elongation status [11]. More importantly, The HA-DOPA hydrogels exhibited excellent tissue adhesion following implantation in a porcine corneal organ [11]. These results demonstrated the promise of this hydrogel as a potential repair material to promote corneal regeneration [11].
5. Conclusions and future perspectives
Click chemistry reactions have been widely used to cross-link polymer networks in hydrogel preparation, owing to their fast kinetics, high-yield properties, chemical selectivity, etc. In this review, we have highlighted various click chemistry reactions commonly used in hydrogel fabrication, including copper-catalyzed azide-alkyne cycloaddition reactions, copper-free click reactions (DA reactions, strain-promoted azide-alkyne cycloaddition reactions, radical mediated thiol-ene reactions, and oxime-forming reactions) and pseudo-click reactions (the thiol-Michael addition reactions and the Schiff base reactions). In addition, we have provided a detailed review of numerous biopolymers, including proteins (e.g. collagen, gelatin, silk fibroin, and mucin), polysaccharides (e.g. hyaluronic acid, alginate, dextran, and chitosan), and polynucleotides (e.g. DNA), going on to describe the click hydrogels formed using the above biopolymers. Based on the chemical and biological properties of the click biopolymer hydrogels, we then presented their medically regenerative applications in terms of skin, bone, spinal cord, cartilage, and corneal healing. This review has focused on the design, fabrication and application of click chemistry-based biopolymeric hydrogels, with the aim of providing scientists with new toolboxes of biomaterials used in click chemical hydrogels.
Although there have been tremendous advances in the design and application of click biopolymeric hydrogels, many fundamental biological, chemical, physical questions still need to be answered. For example, the interactions of click biopolymeric hydrogels within biological environments in vivo require further investigation. The inflammation reactions for click biopolymeric hydrogels and their degraded residues are important but unexploited biological questions. Moreover, click chemistry-based biopolymeric hydrogels for regenerative medicine need to share a comparable modulus with regenerating human tissue. The modulus of the skin, bone, spinal cord, cartilage and cornea measures about 3 × 10° ~ 5 × 102 kPa, 1 × 109 ~ 3 × 109 kPa, 2 ~ 3 kPa, 1 × 107 ~ 9 × 107 kPa and 3 × 103 kPa, respectively (see table 3). On the other hand, the modulus of the click biopolymeric hydrogels used in skin, bone, cartilage and cornea regeneration measures 0.08 ~ 37.3 kPa, 0.1 ~ 5 kPa, 0.5 ~ 13 kPa, 0.48 ~ 28 kPa, respectively (see table 3), far lower than that of human tissue. To meet the mechanical requirements for different tissues, it is equally important to improve the storage and loss modulus of the click biopolymeric hydrogels, for example, by designing double cross-linked or interpenetrating polymeric networks. Last, but certainly not least, the challenge still remains to fully combine laboratory-designed hydrogel matrices with bioactive growth factors, cells, drugs and extracellular vesicles to obtain cost-effective therapies with improved outcomes. Overall, this review may provide new insights for readers in terms of the design of biopolymeric hydrogels for use in regenerative medicine, based on click chemistry reactions.
Table 3. The modulus of the skin, bone, spinal cord, cartilage and cornea, together with that of hydrogels used in the above medical regeneration treatments.
| Types of human tissue | Modulus of human tissue (kPa) | Modulus of the presented hydrogels used in medical regeneration (kPa) |
|---|---|---|
| Skin | 3 × 10° ~ 5 × 102 [158] | 0.08 ~ 3.16 [5]; 21.5 ~ 37.3 [13]; 0.65 ~ 2 [36]; 5 ~ 10 [37] |
| Bone | 1 × 109 ~ 3 × 109 [158] | 1.5 ~ 4 [38]; 0.1 ~ 5 [25] |
| Spinal cord | 2 ~ 3 [158] | / |
| Cartilage | 1 × 107 ~ 9 × 107 [158] | 2 ~ 13 [88]; 0.5 ~ 4 [97] |
| Cornea | 3 × 103 [159] | 1 ~ 28 [12]; 0.48 ~ 0.9 [11] |
Acknowledgments
This work was supported by Natural Science Foundation of Hunan Province (Grant No. 2020JJ5048) and the State Key Laboratory of Molecular Developmental Biology, China (2020-MDB-KF-03). The authors also wish to thank the Fundamental Research Funds of the Central Universities (No. 531118010233).