Abstract
Despite the well-established links between air pollution and human health, vegetation, and aquatic ecosystems, less attention has been paid to the potential impact of reactive atmospheric gases and aerosols on avian species. In this literature review, we summarize findings published since 1950 regarding avian responses to air pollution and discuss knowledge gaps that could be addressed in future studies. We find consistent evidence for adverse health impacts on birds attributable to exposure to gas-phase and particulate air pollutants, including carbon monoxide (CO), ozone (O3), sulfur dioxide (SO2), smoke, and heavy metals, as well as mixtures of urban and industrial emissions. Avian responses to air pollution include respiratory distress and illness, increased detoxification effort, elevated stress levels, immunosuppression, behavioral changes, and impaired reproductive success. Exposure to air pollution may furthermore reduce population density, species diversity, and species richness in bird communities.
Export citation and abstract BibTeX RIS
1. Introduction
It is well established in the peer-reviewed literature that exposure to air pollution causes adverse human health outcomes, including increased risk of respiratory disease, cardiovascular disease, cancer, and mortality (West et al 2016, and references therein). Public health research dating back to the 1950s has established linkages between these adverse health outcomes and respiratory exposure to ambient air pollutants, including tropospheric ozone (O3), aerosols (or particulate matter, PM), nitrogen dioxide (NO2), sulfur dioxide (SO2), carbon monoxide (CO), heavy metals (e.g. lead, mercury), certain hydrocarbons, and other chemically and biologically active species present in the air. Beyond human health, research suggests that all ecosystem types are vulnerable to the effects of air pollution (Lovett et al 2009), with most work to date focused on characterizing the impacts of air pollution on vegetation and aquatic environments. Ozone, in particular, has been shown to reduce rates of photosynthesis in plants (Reich and Amundson 1985), and the acidification of lakes resulting from SO2 and NOx (NO2 + NO) emissions affects both species diversity and species richness in freshwater communities (Brönmark and Hansson 2002).
To date, few studies have explored how chemical characteristics of the air affect non-human animal species, especially in wild populations. In fact, searching the Clarivate Analytics Web of Science platform in November 2016 (referred to hereafter as WoS) for articles related to 'air pollution' and 'human health' published since 1950 produced 2388 results, while searching for articles related to 'air pollution' and 'animals' or 'air pollution' and 'wild populations' produced 658 and 3 results, respectively.
Among non-human animal species, birds may be particularly vulnerable to health-damaging air pollutants. The avian respiratory system, unlike the mammalian respiratory system, is characterized by unidirectional airflow and cross-current gas exchange, features that improve the efficiency of respiration. In fact, birds respire more efficiently than any other type of terrestrial vertebrate. Avian species are therefore more likely to be susceptible to high concentrations of reactive gases and aerosols in the air than mammalian species, and so may serve as useful indicators of air quality (Brown et al 1997). The literature on this topic is limited: WoS searches for articles related to 'air pollution' and 'birds' and 'air quality' and 'birds' produced just 132 and 87 results, respectively. Of the articles found in the latter search, the top three were focused on the effects of poor air quality on poultry, not on wild bird populations. Smith et al acknowledge the lack of understanding of avian responses to inhalation exposure to atmospheric contaminants in a 2007 review article on exposure pathways affecting terrestrial vertebrates (Smith et al 2007).
Birds have long been recognized as sentinel species for environmental change. In the early 20th century, caged canaries were brought down into coal mines to signal when concentrations of toxic gases, such as carbon monoxide, in the mines reached unsafe levels, a practice which led to the use of the colloquial phrase 'canary in the coal mine' to refer to early warning signs of future danger. Rachel Carson's award-winning book Silent Spring, published in 1962, brought attention to the widespread impact of pesticide and insecticide applications on songbirds. A number of avian species have been assessed for their value in the biomonitoring of metals (Burger and Gochfeld 1995, 1997, Saldiva and Böhm 1998, Dauwe et al 2003, Manjula et al 2015) as well as polychlorinated biphenyls and other organic microcontaminants (Arenal et al 2004, Miniero et al 2008). Chronic dietary exposure to heavy metals—including mercury and lead—has been shown to limit avian reproductive success (Scheuhammer 1987, Swiergosz et al 1998), and dietary exposure to dichlorodiphenyltrichloroethane (DDT)—a chemical commonly used in pesticides and insecticides in the mid-20th century—affects calcium metabolism in avian species (Jefferies 1969) and leads to death in songbirds (Wurster et al 1965, Hill et al 1971). Research has also shown that changes in calcium metabolism linked to exposure to DDT results in the production of thin eggshells and the impaired reproductive success of birds of prey (Ratcliffe 1967). Ecologists have also identified a number of responses to climate change in wild bird populations, including phenological shifts in the timing of spring migration and altered species composition of overwintering bird communities (Jones et al 2012, Princé and Zuckerberg 2015). A WoS search for articles related to 'climate change' and 'birds' found 3507 results, with the top paper cited nearly 3800 times.
Birds could also serve as sentinel species for air quality, as they are found globally, in both urban and rural areas, and make use of many different habitat types (Brown et al 1997, Baesse et al 2015). Their wide flight ranges could be beneficial in studying and understanding the spatial distribution of air pollutants (Saldiva and Böhm 1998). Here we present a summary of findings regarding avian responses to air pollution, with an emphasis on inhalation exposure. Our focus on the respiratory system is motivated by the high metabolic rates of birds and expansive literature on human health outcomes associated with inhalation exposure to air pollutants. Below we outline study methods, discuss key findings, and highlight the role of future research. Key findings are divided into five sections: (1) respiratory distress and illness, (2) increased detoxification effort, elevated stress levels, and immunosuppression, (3) behavioral changes, (4) habitat degradation, and (5) impaired reproductive success and demographic consequences. We aim to provide foundational knowledge on avian responses to air pollution, and to support a broader research effort on air pollution impacts on birds.
2. Methods
An initial literature review was performed in January 2016 to gather peer-reviewed studies on avian responses to reactive gases and aerosols. This literature review was performed again in November 2016 to capture more recent publications. Searches were conducted using the Clarivate Analytics WoS platform, accessed through the University of Wisconsin library system. Key search terms included:
| air pollution | air quality | ozone (O3) |
| smog | particulate matter (PM) | PM2.5 |
| PM10 | particulates | aerosols |
| haze | nitrogen dioxide (NO2) | nitric oxide (NO) |
| nitrogen oxides (NOx) | sulfur dioxide (SO2) | carbon monoxide (CO) |
| smoke | soot | metals |
| lead | DDT | mercury |
Each term was paired with the words 'birds' and 'avian' in various combinations, utilizing both the 'title' and 'topic' fields in the basic search function. Over 200 searches were performed. Search results were refined so that only articles or reviews available in English since 1950 were included in the lists of studies generated by WoS. References cited in these studies or citing these studies were also considered, and additional citations were included where appropriate to ensure full coverage of related research. Studies examining poultry-keeping systems were excluded, as these studies did not provide insight into how exposure to outdoor air pollution may affect wild birds. However, laboratory studies designed to investigate the effects of inhalation exposure to ambient air pollutants in birds were included, although some of these studies did use domesticated species, such as chickens, as study specimens.
3. Key findings
To date, three general approaches have been used to characterize avian responses to respiratory exposure to air pollution. Researchers have exposed birds to gaseous mixtures or specific concentrations of gases or aerosols in laboratory experiments, an approach which we refer to as 'controlled exposure.' Birds have also been monitored in natural habitats or caught in the wild for further study, capturing in situ exposure. Finally, we include case studies relevant to avian sensitivity to air pollution. We report key findings below and note which of these three general approaches (controlled exposure, in situ exposure, or case study) was used to elucidate these findings.
3.1. Respiratory distress and illness
Studies have shown that both controlled and in situ exposure to air pollutants cause morphological and physiological changes in the avian respiratory system. Exposure to air pollution clearly causes respiratory distress in birds and increases their susceptibility to respiratory infection.
3.1.1. Controlled exposure
Birds, like other vertebrate species, are susceptible to carbon monoxide poisoning. CO is a product resulting from the incomplete combustion of fossil fuels and biomass. Exposure to CO results in the formation of carboxyhemoglobin (HbCO), limiting the capacity of hemoglobin to transport oxygen to tissues throughout the body (Baker and Tumasonis 1972). Treatment for carbon monoxide poisoning in birds typically includes provision of supplemental oxygen, which reduces the half-life of HbCO (Verstappen and Dorrestein 2005, Simone-Freilicher 2008). The avian carbon monoxide poisoning mechanism was explored in a 1974 study (Tschorn and Fedde 1974), which suggested that CO may depress inhibitory interneurons in the central nervous system, resulting in the abnormal breathing associated with CO poisoning observed in the study specimens.
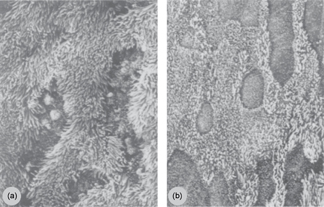
O3 also appears to pose a health risk to birds by causing morphological and physiological changes in the avian respiratory system (Rombout et al 1991, Cuesta et al 2005). Ozone in near-surface environments is chemically formed through reactions involving nitrogen oxides (NOx) and volatile organic compounds (VOCs) in the presence of sunlight. In a study by Rombout et al (1991), adult male Japanese quail (Coturnix coturnix japonica) were exposed to varying O3 concentrations. Exposure to 0.50 ppm O3 resulted in the reduction and shortening of cilia in the trachea and bronchi (figure 1), hypertrophy in the secondary bronchi, hemorrhaging and inflammation in the capillary network, necrosis of epithelial cells lining the air capillaries, and symptoms of pulmonary edema. Quail exposed to higher concentrations of O3 (1.50 ppm) were more severely affected. Individuals in this treatment group showed a dramatic reduction in the number of ciliated cells in the trachea and bronchi. Hemorrhaging and changes in the structure of the atrial epithelium resulted in the closing of the atrial space, reducing air flow and possibly contributing to the labored breathing exhibited by this treatment group during the experiment. Only the birds in this treatment group presented a statistically significant increase in lung weight and lactate dehydrogenase (LDH) activity (Rombout et al 1991), often considered a response to injury or stress. Cuesta et al (2005) also found a statistically significant increase in expression of adrenomedullin-like protein in pigeons (Columba livia domestica) exposed to high levels of O3 as compared to those in a control group, which appears to be the only study to date on air pollution impacts on adrenomedullin-like immunoreactivity in birds, a sign of respiratory distress.
Figure 1. Standard electron microscopy (2000×) of the epithelium of a secondary bronchus in (a) a Japanese quail exposed to 0.0 ppm O3 and (b) a Japanese quail exposed to 0.50 ppm O3. Note the reduction and shortening of cilia in the individual exposed to O3. Imagery from Rombout et al 1991, copyright © 1991 published by Elsevier Inc.
Download figure:
Standard image High-resolution imageExposure to SO2 may impair the avian immune response to inhaled antigens, making birds more susceptible to disease (Wakabayashi et al 1977). SO2 is a gas that is emitted from the combustion of sulfur-containing fuels, such as coal or petroleum oil. Results from a 1977 study show that exposure to SO2, even at concentrations as low as 1.4 ppm, compromises the effectiveness of the mucocilliary system in White Leghorn chickens, which plays a crucial role in reducing the incidence of respiratory disease by clearing contaminants, including infectious agents, from the airway (Wakabayashi et al 1977). Fedde and Kuhlmann (1979) found that White Leghorn chickens exposed to much higher concentrations of SO2 (>1000 ppm) exhibited signs of respiratory distress, and the majority of those exposed to SO2 at a concentration of 5000 ppm died.
Multiple studies have shown that birds are also likely affected by elevated concentrations of particle pollution (Sterner 1993a, 1993b, Tell et al 2006, Tell et al 2012). PM includes any liquid or solid suspended in the air, typically classified by size such that PM2.5 represents PM less than 2.5 microns in diameter and PM10 represents PM less than 10 microns in diameter. Health-relevant PM can include directly emitted particles, such as wind-blown dust or smoke from fires, and/or chemically formed particles, such as sulfate and nitrate aerosols. Tell et al (2006) examined the use of an aerosolized fluorescent microsphere technique of their own design to study particle deposition in adult domestic pigeons. Their results demonstrated that smaller particles (those less than three microns in diameter) are more likely to be well dispersed in the avian respiratory system than larger particles (those greater than six microns in diameter), which are more likely to remain in the upper airway (Tell et al 2006). Tell et al (2012) confirmed that the extent of deposition increases with exposure time (Tell et al 2012). Understanding how particles are deposited in the respiratory system and cleared from the airway will be important in differentiating responses to acute and chronic exposure to air pollution.
3.1.2. In situ exposure
A number of studies have used microscopic imagery and chemical analysis to compare the morphology and biochemistry of lung tissue from birds captured in urban and rural areas in order to discern physiological differences that are likely attributable to urban air pollution (Lorz and López 1997, Cuesta et al 2005, Sicolo et al 2009, Ejaz et al 2014, Steyn and Maina 2015). Birds exposed to urban air pollution may exhibit a buildup of cellular and mineral debris leading to lung parenchymal consolidation or alveolar consolidation, a characteristic condition of pneumonia (Ejaz et al 2014). Researchers in Spain have shown that exposure to urban air pollution may also impact the avian pulmonary surfactant system (Lorz and López 1997, Cuesta et al 2005).
Pulmonary surfactant is a lipoprotein complex, and the secretion of pulmonary surfactant helps reduce tension in the respiratory system, thus preventing the lungs from collapsing during expiration (Lorz and López 1997, Cuesta et al 2005). Pulmonary surfactant also plays an important role in the defense mechanisms of the lung. The lipids and proteins that make up the surfactant are stored in lamellar bodies—dense structures found in the cytoplasm of type 2 pneumocytes (Lorz and López 1997). In a 1997 study, pigeons were captured and examined to determine if the number of lamellar bodies in lung tissue differed between birds living in rural habitats (collected from a farm in the village of Guadalajara) and urban habitats (captured in the city of Madrid). If more pulmonary surfactant is excreted by type 2 pneumocytes, the number of lamellar bodies would be expected to decline. The researchers found a 33% reduction in the quantity of lamellar bodies in urban birds as compared to rural birds, indicative of elevated secretion of pulmonary surfactant and, therefore, respiratory distress (Lorz and López 1997).
Phosphatidylcholine is an important component of pulmonary surfactant. In mammals, secretion of phosphatidylcholine is stimulated by adrenomedullin, a peptide hormone. Elevated concentrations of adrenomedullin have been documented in the pathologies for several mammalian respiratory diseases, including asthma. A 2005 study demonstrated the presence of an adrenomedullin-like protein in the avian respiratory system (Cuesta et al 2005). In this study, pigeons were captured and observed to determine if expression of this adrenomedullin-like protein differed between birds living in rural and urban habitats. Results showed that expression of the adrenomedullin-like protein was higher in birds exposed to chronic urban air pollution, evidence of greater stimulation of phosphatidylcholine secretion and elevated levels of pulmonary surfactant. This finding suggests that pigeons exposed to urban air pollution suffer from respiratory distress (Cuesta et al 2005).
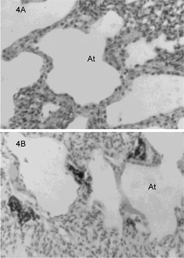
In Johannesburg, South Africa, Steyn and Maina (2015) found that house sparrows (Passer domesticus), Cape glossy starlings (Lamprotornis nitens), and laughing doves (Streptopelia senegalensis) exposed to urban air pollution had a greater number of free surface macrophages in the lungs than those from rural areas. Macrophages play an important role in immune response by destroying or sequestering foreign particulates and aerosols. The doves exhibited the greatest numbers of macrophages, followed by the starlings, while the sparrows had the least (Steyn and Maina 2015). These results align with observations by Lorz and López (1997), which demonstrated that domestic pigeons from urban areas had a greater number of macrophages in lung tissue than those from rural sites (figure 2).
Figure 2. Images of atrial lung tissue harvested from (a) rural pigeons and (b) urban pigeons produced using light microscopy (240×). Note the clustering of macrophages near the atrial space in the birds exposed to higher levels of urban air pollution. Imagery from Lorz and López 1997, copyright © 1997 Wiley-Liss, Inc.
Download figure:
Standard image High-resolution imageA series of studies conducted at the University of Barcelona in the 1990s sought to investigate changes in the tracheal epithelium of birds exposed to SO2, NOx, and particulate emissions from coal-fired power plants in northeastern Spain (Llacuna et al 1993, 1996, Gorriz et al 1994). Goldfinches (Carduelis carduelis) exposed to emissions from a power plant showed greater mucus production, a shortening of the cilia in the epithelial cells lining the trachea, and an increase in the number and size of secretory vesicles. These changes to the tracheal epithelium were less pronounced in rock buntings (Emberiza cia) and great tits (Parus major), which the authors suggest may be attributable to the study design which prevented the goldfinches, trapped in cages, from moving away from the source of emissions (Llacuna et al 1993). A related study found abnormal orientation of the cilia in rock buntings and great tits exposed to emissions from a power plant, possibly resulting from increased production of mucus, but not in goldfinches or blackbirds (Turdus merula). Normal orientation of cilia helps to clear particles from the airway and support air flow (Gorriz et al 1994).
3.1.3. Case studies
Smoke is known to cause both thermal and chemical damage to avian lung tissue, as well as increase a bird's susceptibility to respiratory infection (Morris et al 1986, Verstappen and Dorrestein 2005, Simone-Freilicher 2008, Kinne et al 2010). Morris et al (1986) linked exposure to smoke with an outbreak of the contagious respiratory disease laryngotracheitis in a flock of chickens, as well as a short-term increase in mortality and a short-term decline in egg production. Verstappen and Dorrestein (2005) documented the effects of indoor smoke exposure in blue-fronted Amazon parrots (Amazona aestiva aestiva) kept in an aviary. Within hours following exposure, the parrots developed dyspnea, pulmonary edema, and minor damage to their lung tissue (Verstappen and Dorrestein 2005). A female ruby blue-headed pionus parrot (Pionus menstruus rubrigularis) showed similar symptoms, as well as weight loss, secondary respiratory infections, and decreased activity after repeated incidences of indoor smoke exposure (Simone-Freilicher 2008). Kinne et al (2010) suggest that carbon monoxide poisoning is usually the cause of death in birds following smoke exposure, but that mycotic air sacculitis, pneumonia, and additional responses to other compounds present in smoke may continue for days or weeks.
Smoke inhalation may also compromise the ability of birds to escape during wildfire events. While the eggs and chicks of ground-nesting species and waterfowl undergoing molt are thought to be most susceptible to fire events, monitoring of wading bird species in the Everglades showed that even adult, flighted birds may die during wildfires. A fire in April of 1999 caused the death of 50 adult white ibises (Eudocimus albus) found on a cattail island. The ibises likely became trapped due to the presence of thick smoke (Epanchin et al 2002).
3.2. Increased detoxification effort, elevated stress levels, and immunosuppression
Laboratory and field studies have also determined that exposure to air pollutants results in increased detoxification effort and elevated stress levels in birds, providing further evidence of the negative health impacts of air pollution on avian species. This research also shows that the avian immune response may be impaired following acute or chronic exposure to air pollution, giving added weight to the previous discussion of how birds exposed to health-damaging air pollutants may be more susceptible to respiratory disease (see section 3.1).
3.2.1. Controlled exposure
Inhalation exposure to air pollution may increase the stress response and detoxification effort in birds (Cruz-Martinez et al 2015b). SO2, NO2, and VOCs are the major air pollutants associated with oil sands operations, an important point source of air pollution. VOCs are a class of chemicals that contain carbon and hydrogen, many of which are associated with direct human health impacts, especially cancer. To study how emissions from oil sands operations may impact avifauna, researchers exposed Japanese quail and American kestrels (Falco sparverius) to gaseous mixtures of SO2, NO2, and VOCs in whole-body inhalation chambers and measured resulting corticosterone (CORT) and 7-ethoxyresorufin-O-dealkylase (EROD) concentrations in blood samples. CORT activity is an indicator of stress, while EROD activity is often used to signal the body's increased detoxification effort, especially of polycyclic aromatic hydrocarbons.
Japanese quail were randomly assigned to three groups: control (hydrocarbon-free air), low exposure (0.5 ppm SO2, 0.2 ppm NO2, 0.6 ppm benzene, and 1 ppm toluene), and high exposure (50 ppm SO2, 20 ppm NO2, 60 ppm benzene, and 100 ppm toluene). Quail were exposed to these gaseous mixtures for 1.5 h each day for 20 d. American kestrels were separated into a control and experimental group and exposed to hydrocarbon-free air and 5.6 ppm SO2, 2 ppm NO2, 0.6 ppm benzene, and 1 ppm toluene, respectively, for 1.4 h each day for 18 d (Cruz-Martinez et al 2015b).
CORT activity increased and EROD activity doubled in the kestrels exposed to experimental conditions compared to those exposed to hydrocarbon-free air, though these results were not statistically significant. CORT activity increased in the quail in the low exposure group as compared to the control and high exposure groups. These results are characteristic of a hormetic response to toxins, in which low doses of toxins prompt a response that is inhibited at higher doses. There was no statistically significant difference in the detoxification effort amongst quail in exposure trials. Quail, like other species in the Galliformes order, may be more resistant than other avian species to toxic gases (Cruz-Martinez et al 2015b). Though the results of this study demonstrate a specific physiological response to elevated levels of common atmospheric contaminants, it is not possible to isolate the response to any of the four distinct compounds the quail and kestrels were exposed to in these trials (Cruz-Martinez et al 2015b). Fernie et al (2016) also show that exposure to a gaseous mixture of benzene, toluene, NO2, and SO2 might upregulate function of the hypothalamus-pituitary-thyroid axis in kestrels, which could affect the hormonal control of many key activities, including metabolism and reproduction. Additional research is needed to further characterize the role of the thyroid gland in the stress response and detoxification effort in birds following exposure to air pollution (Fernie et al 2016).
According to a study conducted in 2005 and 2006, exposure to VOCs may compromise the avian immune system (Olsgard et al 2008). In 2005, American kestrels caught in the wild near Prince Albert, Saskatchewan, Alberta were divided into two groups: control (breathing grade air) and high exposure (10 ppm benzene and 80 ppm toluene). The kestrels were exposed to these gaseous mixtures for 1 h each day for 28 d. In 2006, captive kestrels were assigned to one of three groups: control (breathing grade air), low exposure (0.1 ppm benzene and 0.8 ppm toluene), and high exposure (10 ppm benzene and 80 ppm toluene). The kestrels were exposed to these gaseous mixtures for 1.5 h each day for 27 d. The results of this study indicate that while the humoral immune response might not be impacted by inhalation exposure to VOCs, the cell-mediated immune response is suppressed in kestrels exposed to gaseous mixtures of benzene and toluene, at both the low dose and high dose levels (Olsgard et al 2008). These findings support earlier studies indicating that exposure to air pollution may increase the risk of respiratory infection in birds.
3.2.2. In situ exposure
Exposure to industrial air pollution has been linked to genotoxic effects in birds (Baesse et al 2015), including higher rates of heritable genetic mutations (Yauk and Quinn 1996, Yauk et al 2000, King et al 2014). Exposure to air pollution has also been associated with the bioaccumulation of metals in the tissues of vital organs of birds that occupy habitats near point sources of air pollution (Nyholm 1994, 1995, Llacuna et al 1995, Hui 2002, Lovett et al 2009, Berglund et al 2011) or habitats affected by long-range transport of air pollution (Nybø et al 1996). Exposure to metals may lead to oxidative stress in some birds (Koivula and Eeva 2010, Rainio et al 2013). Avian exposure to mercury is correlated with neurological damage and impaired reproductive success (Lovett et al 2009) while the uptake of lead has been shown to affect the behavioral development of chicks and negatively impact their growth and survival rates (Burger and Gochfeld 2000). Exposure to lead also reduces the activity of δ-aminolevulinic acid dehydratase (ALAD), an enzyme that plays a crucial role in the production of heme. Findings from a study of European starlings (Sturnus vulgaris) in Prince George's County, Maryland, United States linked respiratory and dietary exposure to lead emitted by motor vehicles to decreased ALAD activity (Grue et al 1986).
A 1996 study focused on characterizing hematological changes in great tits, rock buntings, and blackbirds exposed to emissions from power plants (Llacuna et al 1996). The study found that exposure to industrial emissions from a coal-fired power plant decreased erythrocyte (red blood cell) counts by 11.4% in great tits and by 16.2% in rock buntings. Erythrocytes were also noticeably larger. This is likely due to the immune system's targeted destruction of defective erythrocytes and rapid production of new, immature erythrocytes, which are bigger. Blackbirds also showed statistically significant weight loss and greater concentrations of transaminases, a symptom indicative of liver disease. Plasma samples from rock buntings captured near the power plant also showed a decrease in beta globulins, indicative of infection (Llacuna et al 1996).
Industrial air pollution associated with oil sands activity may also negatively affect the health of avifauna. A recent study by Cruz-Martinez et al studied detoxification effort and immune response in tree swallow nestlings (Tachycineta bicolor) at sites near oil sands operations (2015a). Exposure to elevated concentrations of ambient air pollutants associated with oil sands activity was linked to increased detoxification effort and suppression of cell-mediated immunity (Cruz-Martinez et al 2015a).
Exposure to high levels of urban air pollution was found to increase concentrations of elemental toxins in the tissues of vital organs in wild birds, including starlings, owls, crows, and pigeons, captured in Lahore, Pakistan. The magnitude of this increase varied by tissue type and species. Researchers also noted symptoms of degeneration and necrosis of liver tissue in captured birds (Ejaz et al 2014).
Findings from a study of pigeons in the city of Milan, Italy indicate that porphyrin concentrations in bird excrement and blood methemoglobin levels may serve as useful bioindicators of urban air pollution. Results from this study showed that excreta collected from the pigeons exposed to urban air pollution had significantly higher total porphyrin concentrations and greater proportions of protoporphyrins compared to that collected from control pigeons. Methemoglobin levels were also higher in blood samples collected from the urban birds. This was one of the few studies we discuss in our review that included measurements of ambient air quality, which allowed the researchers to link increased protoporphyrin concentrations in excreta to elevated concentrations of polycyclic aromatic hydrocarbons and higher blood methemoglobin levels to elevated concentrations of ozone and benzene (Sicolo et al 2009).
3.3. Behavioral changes
Few studies have assessed how exposure to air pollutants may alter bird behavior, but existing research suggests that many different behaviors could be affected, from spontaneous activity to homing.
3.3.1. Controlled exposure
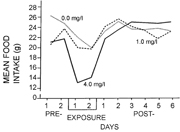
Avian exposure to phosphoric acids aerosols may result in changes in activity and weight loss (Sterner 1993a, 1993b). A series of studies conducted by Sterner in 1993 was designed to determine if acute exposure to phosphoric acids aerosols affected spontaneous activity such as walking and preening in rock doves (Columba livia). Twenty-four rock doves (11 males and 13 females) were exposed to phosphoric acids aerosols at concentrations of 0.0, 1.0, and 4.0 mg l−1 for 80 min day−1 for two days. Preening and ambulatory activity were found to significantly decline in the 4.0 mg l−1 group following the first exposure (Sterner 1993a). Sterner's studies also determined that rock doves exposed to phosphoric acids aerosols at concentrations of 1.0 mg l−1 and 4.0 mg l−1 exhibited reduced water intake, food intake, and body weight as compared to control groups, with recovery dependent on both concentration and sex (figure 3) (Sterner 1993b).
Figure 3. Plot showing mean food intake by rock doves in grams during the pre-exposure (2 d), exposure (2 d), and post-exposure (6 d) periods. The lines represent results from different treatment groups (dotted line: 0.0 mg l−1, dashed line: 1.0 mg l−1, solid line: 4.0 mg l−1). Note that food intake declines in each treatment group, but drops most significantly in birds exposed to the highest level of phosphoric acids aerosols. Recovery in the post-exposure phase depends on concentration. Plot from Sterner 1993b, permission to reuse this figure obtained from Taylor & Francis Ltd (www.tandfonline.com).
Download figure:
Standard image High-resolution image3.3.2. In situ exposure
A recent study also established a connection between behavioral changes and exposure to particulate pollution in cities. It is possible that high concentrations of particulate matter resulting in the haze seen in metropolitan centers may motivate city pigeons to home faster. Further study is needed to determine if this change in behavior is seen in other cities or as a result of exposure to different types of air pollutants (Li et al 2016).
3.4. Habitat degradation
Industrial emissions often include NOx, SO2, and heavy metals. Emitted NOx and/or SO2 react in the atmosphere to form nitric and sulfuric acid, which when deposited in wet or dry form contribute to acid deposition (commonly referred to as 'acid rain') and soil acidification. Acid deposition has been hypothesized to contribute to the bioaccumulation of metals in birds (Scheuhammer 1991), possibly by increasing the solubility of elements such as aluminum (Scanes and McNabb 2003). Exposure to acid rain also affects calcium and phosphorus metabolism, production of stress hormones, food intake, growth rate, and reproductive success (Scanes and McNabb 2003). Acid deposition and heavy metal uptake of soils near point sources of industrial emissions often affect the composition of the plant and invertebrate communities on which birds depend for their food supply (Eeva et al 1998, 2003, 2005, Belskii and Belskaya 2009, 2013c, Costa et al 2011, Belskii and Grebennikov 2014). Changes in the chemical environment may promote ecological shifts that increase food availability for some species. For example, great tits may have greater reproductive success at sites near point sources of emissions associated with the pulp and paper industry due to higher abundance of caterpillars—a key food resource for this species—in these areas (Costa et al 2011, 2014). However, other avian species may suffer if air pollution reduces the quantity or quality of food resources (Eeva et al 2003, 2005, Belskii and Grebennikov 2014).
Changes in food supply may also result in a decrease in the availability of foods bearing pigments (i.e. carotenoids), which in turn could indirectly affect reproductive success by reducing the vibrancy of a bird's plumage. Faded plumage is a disadvantage for males, as more vibrant, colorful plumage appears to be a sign of good physical condition, rendering males more attractive to potential female mates (Eeva et al 1998). The antioxidant activity of pigments also helps to protect birds from oxidative stress.
Food intake may also be affected by behavioral changes that limit foraging time. Birds may spend less time foraging if tree canopies thin as a result of air pollution, making birds more susceptible to predation (Brotons et al 1998). Changes in forest structure or the composition of local habitat may also affect the prevalence of ectoparasites, which could affect the health of both nestlings and adult birds (Belskii et al 2005, Eeva and Klemola 2013).
3.5. Impaired reproductive success and demographic consequences
Both the direct, toxic effects of exposure to atmospheric contaminants as well as the indirect effects of air pollution on avifauna (i.e. shifts in food availability) have been linked to impaired reproductive success. Reproductive success is a measure of how effective a parent generation is in passing their genes on to subsequent generations, and as such is often assessed by determining not only the number of offspring produced but also the fitness of those offspring.
3.5.1. Controlled exposure
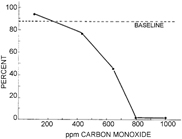
While previous studies had established that embryos do respond to their gaseous environments, a 1972 study provided further insight into how CO diffusion through the eggshell and inner and outer shell membranes may affect the growth and development of bird embryos. Baker and Tumasonis (1972) found that the hatchability and viability of White Leghorn chicken eggs declined as CO levels increased (figure 4), and 425 ppm CO was determined to be the critical concentration for these parameters. Vasodilation of blood vessels was noted in embryos exposed to CO concentrations above 425 ppm. LDH concentrations in the blood serum also increased in embryos exposed to CO at the critical level, a common indicator of injury or stress. Exposure to higher concentrations of CO also resulted in increased mortality, and exposure to 1000 ppm CO resulted in 100% mortality. Chicks that hatched following exposure to CO were smaller than control chicks and showed physical abnormalities if exposed during the embryonic stage to CO concentrations above 425 ppm (Baker and Tumasonis 1972). Furthermore, the results of this study show that while the eggshell and inner and outer shell membranes of White Leghorn chicken eggs limit the diffusion of CO, the ability of these layers to protect embryos from CO poisoning appears to degrade with time. Both the age of the egg and the shell condition therefore affect the permeability of an embryonated egg (Baker and Tumasonis 1972). If the eggshell and inner and outer shell membranes of eggs provide only limited protection against elevated concentrations of CO, it is plausible that other atmospheric contaminants might also be able to diffuse through the egg's layered surface and affect bird embryos.
Figure 4. Plot from Baker and Tumasonis 1972, showing percent hatchability as a function of carbon monoxide concentration. As carbon monoxide concentrations increase, hatchability declines. Permission to reuse this figure obtained from Taylor & Francis Ltd (www.tandfonline.com).
Download figure:
Standard image High-resolution image3.5.2. In situ exposure
Recent studies in Finland, (Eeva and Lehikoinen 1995, 1996), Russia (Belskii et al 1995a, 1995b, 2005), Belgium (Dauwe et al 2005), Sweden, (Nyholm 1994, 1995), Portugal (Costa et al 2011, 2014), and Tunisia (Alaya-Ltifi et al 2012) have examined the reproductive success of birds living within industrial air pollution gradients, as well as the fitness of their offspring. It should be noted that because these field studies examined how proximity to point sources of air pollution impacts reproductive success, any observed effects could be due to habitat degradation, inhalation exposure to atmospheric contaminants, and/or ingestion of pollutants that deposit to Earth's surface and are consumed with food.
As part of an ongoing study first begun in the 1990s, researchers observed pied flycatchers (Ficedula hypoleuca), a migratory species, and great tits, a resident species, at nest boxes constructed at 14 study sites located within an elliptical air pollution gradient surrounding a copper-smelter complex in Harjavalta, Finland. The research team used the heavy metal content of the soil at this industrial site as a proxy measurement for total air pollution exposure associated with operation of the smelter, known to emit SO2 and heavy metals (copper, lead, and nickel). The heavy metal content decreased exponentially with distance from the smelter. The researchers wanted to determine if the reproductive success of these birds was impaired at study sites closer to the copper smelter due to the production of low-quality eggs and increased nestling mortality. This hypothesis was based on previous work showing that exposure to heavy metals and acidifying compounds might impair avian reproductive success by affecting calcium metabolism and reducing the availability of calcium in local food resources, all of which results in eggs with thinner shells and growth abnormalities in nestlings (Scheuhammer 1991, Eeva and Lehikoinen 1995, 1996).
Both the pied flycatchers and the great tits occupying nest boxes at sites closest to the smelter laid fewer eggs, but the pied flycatcher showed an overall greater vulnerability to exposure to industrial air pollution than did the great tit. Hatching success of pied flycatchers was also dramatically reduced at sites closest to the complex (Eeva and Lehikoinen 1995). Production of low-quality eggs did appear to drive the impairment in reproductive success at this industrial site. The eggs of the pied flycatchers were 8% smaller by volume with 17% thinner shells at sites closest to the factory complex as compared to sites located 10 km away, and microscopy revealed that the surface of the eggs collected from sites nearest the factory were more rough and porous (Eeva and Lehikoinen 1995). The foraging strategy of the pied flycatcher was suggested to account for its greater sensitivity to industrial pollutants (Eeva and Lehikoinen 1995). Later work by this group also found that pied flycatchers, unlike great tits, exhibit stress responses suggestive of a direct toxic effect in the vicinity of point sources of industrial air pollution (Eeva et al 2000). Species-specific differences in avian responses to air pollution have been noted in other studies as well. For example, researchers in Flanders, Belgium were surprised to find no statistically significant differences in the breeding performance of blue tits (Parus caeruleus) within an industrial air pollution gradient resulting from the operation of a metallurgic smelter (Dauwe et al 2005).
The impacts of heavy metal and SO2 emissions from a copper-smelting plant on pied flycatchers were also studied in Russia by Belskii et al (1995a, 1995b, 2005). Results from these studies indicate that reproductive success is impaired at nesting sites closest to point sources of industrial emissions (Belskii et al 1995a, 1995b, 2005). At nests more than 15 km from the copper smelter, clutch size increased by a factor of 1.5, and both the number of hatched chicks and the number of fledglings per nest doubled compared to nests located in the nearby vicinity of the plant. At those sites closest to the plant, egg mortality was also 3.5 times greater (Belskii et al 2005). In addition, the results of this study showed that the proportion of nestlings infested by parasitic fly larvae as well as the severity of infestation (as measured by the average number of fly larvae per infested chick) increased with decreasing distance to the plant. Higher liver indices, reduced hemoglobin concentrations, and greater proportions of immature erythrocytes in nestlings were linked to both the direct toxic effect of air pollutants and greater incidence and severity of parasitic infestation, leading the authors to suggest that exposure to industrial air pollution leads to a general weakening in nestlings, rendering them more susceptible to infestation and subsequent infection (Belskii et al 2005).
Fortunately, Eeva and Lehikoinen (2000, 2015) show that reducing industrial emissions from point sources improves the reproductive success of birds living nearby. As emissions at the copper smelter in Harjavalta declined, both the clutch size of pied flycatchers and the number of fledglings per nest increased (Eeva and Lehikoinen 2000, 2015). However, recovery is often slow. Even as emissions and the resulting atmospheric deposition of heavy metals decline, birds are still exposed to high concentrations of metals in soils due to the much more gradual rate of soil cycling (Berglund and Nyholm 2011).
Research suggests that impaired reproductive success in birds resulting from exposure to air pollution may also have demographic consequences. Several studies have linked declines in bird population density (Flousek 1989, Eeva et al 2002, 2012, Saha and Padhy 2011, Belskii and Belskaya 2013a, 2013b, Alaya-Ltifi and Selmi 2014), species diversity (Saha and Padhy 2011, Eeva et al 2012, Belskii and Belskaya 2013a, 2013b, Alaya-Ltifi and Selmi 2014), and species richness (Belskii and Lyakhov 2003, Belskii and Belskaya 2013a, 2013b) to exposure to industrial air pollution. As proximity to a point source of industrial air pollution increases, population density, species diversity, and species richness decrease. Researchers point out that changes in the structure and composition of soils and vegetation resulting from air pollution may affect a number of variables important to the life histories of the birds studied, including food supply and the availability of nesting sites. None of these studies causally linked inhalation exposure to industrial air pollutants to changes in population density, species diversity, or species richness. Within an air pollution gradient, it is difficult to determine how much of the variation in these metrics is due to direct exposure to airborne toxins and how much is due to other abiotic and biotic changes associated with industrial pollution in the local environment (Belskii and Belskaya 2013b). Noise pollution may also be a confounding variable in assessing the impacts of industrial air pollution on birds, as noise pollution often increases with proximity to industrial sites (Saha and Padhy 2011).
It is possible that species-specific differences in reproductive success following exposure to air pollution could lead to shifts in the composition of avian communities. Studies have already linked changes in avian community composition to habitat degradation caused by air pollution. Damage caused by air pollution to trees alters the structure of forest ecosystems. While some birds may be able to colonize these habitats, others may make use of ecological niches that are no longer available within that landscape. Over time, this may change the community composition of forests exposed to industrial air pollution (Capek 1991, Capek et al 1998, Belskii and Lyakhov 2003, Eeva et al 2012, Belskii and Belskaya 2013a). For example, point counts of birds in an air pollution gradient resulting from industrial emissions from the Karabash Copper Smelter in Russia showed that the proportion of canopy-nesting and ground-nesting species in the area increased along the gradient while the proportion of hole-nesting species decreased (Belskii and Belskaya 2013a).
4. Discussion
This review finds clear evidence that birds are affected by exposure to a range of reactive gases and particles in the air, including air pollutants with established adverse impacts on human health. Avian responses to air pollution include respiratory distress and illness, increased detoxification effort, elevated stress levels, immunosuppression, behavioral changes, and impaired reproductive success. Air pollution may also reduce population density, species diversity, and species richness in bird communities (table 1, figure 5). These demographic consequences are a result of both the direct, toxic effect of exposure to air pollution as well as habitat degradation resulting from poor air quality. Although available evidence consistently demonstrates that exposure to air pollution impacts birds, the total number of studies on this topic is limited, and important gaps remain.
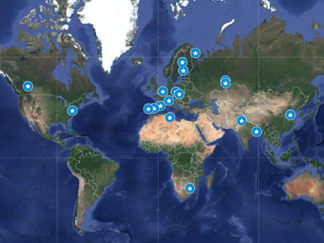
Table 1. This table lists the field and laboratory studies cited in section 3, key findings that characterize avian sensitivity to air pollution. Entries are categorized by both the type of response explored (i.e. respiratory distress and illness; increased detoxification effort, elevated stress levels, and immunosuppression; behavioral changes; habitat degradation; or impaired reproductive success and demographic consequences) and the type of exposure study specimens were subjected to (i.e. controlled or in situ). An asterisk (⁎) is used to identify studies focused on examining community-level responses, such as species diversity, species richness, or community composition. A map showing the locations where birds in these studies were exposed to air pollution in situ is included in figure 5.
| Response | Exposure | Study | Species | Location of in situ exposure |
|---|---|---|---|---|
| Respiratory distress and illness | Controlled exposure | Baker and Tumasonis 1972 | White Leghorn chickens | n/a |
| Tschorn and Fedde 1974 | White Leghorn chickens | n/a | ||
| Wakabayashi et al 1977 | White Leghorn chickens | n/a | ||
| Fedde and Kuhlmann 1979 | White Leghorn chickens | n/a | ||
| Rombout et al 1991 | Japanese quail | n/a | ||
| Cuesta et al 2005 | pigeons | n/a | ||
| In situ exposure | Llacuna et al 1993 | rock buntings, great tits, and goldfinches | Cercs, Spain | |
| Gorriz et al 1994 | rock buntings, coal tits, blackbirds, and goldfinches | Cercs, Spain | ||
| Lorz and López 1997 | pigeons | Madrid, Spain | ||
| Cuesta et al 2005 | pigeons | Madrid, Spain | ||
| Ejaz et al 2014 | starlings, owls, crows, and pigeons | Lahore, Pakistan | ||
| Steyn and Maina 2015 | house sparrows, Cape glossy starlings, and laughing doves | Johannesburg, South Africa | ||
| Increased detoxification effort, elevated stress levels, and immunosuppression | Controlled exposure | Olsgard et al 2008 | American kestrels | n/a |
| Cruz-Martinez et al 2015b | American kestrels and Japanese quail | n/a | ||
| Fernie et al 2016 | American kestrels | n/a | ||
| In situ exposure | Grue et al 1986 | European starlings | Prince George's County, Maryland, United States | |
| Llacuna et al 1996 | great tits, rock buntings, and blackbirds | Cercs, Spain | ||
| Sicolo et al 2009 | pigeons | Milan, Italy | ||
| Berglund and Nyholm 2011 | pied flycatchers | Skelleftehamn, Sweden | ||
| Rainio et al 2013 | great tits, blue tits, and pied flycatchers | Harjavalta, Finland | ||
| Ejaz et al 2014 | starlings, owls, crows, and pigeons | Lahore, Pakistan | ||
| Cruz-Martinez et al 2015a | tree swallows | Alberta, Canada | ||
| Behavioral changes | Controlled exposure | Sterner 1993a | rock doves | n/a |
| Sterner 1993b | rock doves | n/a | ||
| In situ exposure | Li et al 2016 | pigeons | North China Plain | |
| Habitat degradation | Brotons et al 1998 | crested tits, long-tailed tits, and coal tits | Cercs, Spain | |
| Eeva et al 2003 | great tits | Harjavalta, Finland | ||
| In situ exposure | Belskii et al 2005 | pied flycatchers | Revda, Sverdlovsk Oblast, Russia | |
| Eeva et al 2005 | great tits and pied flycatchers | Harjavalta, Finland | ||
| Belskii and Belskaya 2009 | pied flycatchers | Revda, Sverdlovsk Oblast, Russia | ||
| Costa et al 2011 | great tits | Figueira da Foz, Portugal | ||
| Belskii and Belskaya 2013c | pied flycatchers and coal tits | Revda, Sverdlovsk Oblast, Russia | ||
| Eeva and Klemola 2013 | pied flycatchers | Harjavalta, Finland | ||
| Belskii and Grebennikov 2014 | pied flycatchers | Revda, Sverdlovsk Oblast, Russia | ||
| Costa et al 2014 | great tits | Figueira da Foz, Portugal | ||
| Impaired reproductive success and demographic consequences | Controlled exposure | Baker and Tumasonis 1972 | White Leghorn chickens | n/a |
| In situ exposure | Flousek 1989⁎ | multiple | Giant Mountains, Czech Republic | |
| Capek 1991⁎ | multiple | Moravskoslezské Beskydy Mountains, Czech Republic | ||
| Nyholm 1994 | pied flycatchers | Skelleftehamn, Sweden | ||
| Belskii et al 1995a | great tits, coal tits, and pied flycatchers | Revda, Sverdlovsk Oblast, Russia | ||
| Belskii et al 1995b | great tits, coal tits, and pied flycatchers | Revda, Sverdlovsk Oblast, Russia | ||
| Eeva and Lehikoinen 1995 | great tits and pied flycatchers | Harjavalta, Finland | ||
| Nyholm 1995 | pied flycatchers | Skelleftehamn, Sweden | ||
| Eeva and Lehikoinen 1996 | great tits and pied flycatchers | Harjavalta, Finland | ||
| Capek et al 1998⁎ | multiple | Moravskoslezské Beskydy Mountains Czech Republic | ||
| Eeva et al 1998 | great tits | Harjavalta, Finland | ||
| Eeva and Lehikoinen 2000 | great tits and pied flycatchers | Harjavalta, Finland | ||
| Eeva et al 2000 | great tits and pied flycatchers | Harjavalta, Finland | ||
| Eeva et al 2002⁎ | multiple | Harjavalta, Finland | ||
| Belskii and Lyakhov 2003⁎ | multiple | Revda, Sverdlovsk Oblast, Russia | ||
| Belskii et al 2005 | pied flycatchers | Revda, Sverdlovsk Oblast, Russia | ||
| Dauwe et al 2005 | blue tits | Flanders, Belgium | ||
| Berglund and Nyholm 2011 | pied flycatchers | Skelleftehamn, Sweden | ||
| Costa et al 2011 | great tits | Figueira da Foz, Portugal | ||
| Saha and Padhy 2011⁎ | multiple | West Bengal, India | ||
| Alaya-Ltifi et al 2012 | rufous bush robins, blackbirds, Orphean warblers, and woodchat shrikes | Gabès, Tunisia | ||
| Eeva et al 2012⁎ | multiple | Harjavalta, Finland; Karabash, Russia; Monchegorsk, Russia; and Revda, Russia | ||
| Belskii and Belskaya 2013a, 2013b⁎ | multiple | Karabash, Russia | ||
| Alaya-Ltifi and Selmi 2014⁎ | multiple | Gabès, Tunisia | ||
| Costa et al 2014 | great tits | Figueira da Foz, Portugal | ||
| Eeva and Lehikoinen 2015 | pied flycatchers | Harjavalta, Finland |
Figure 5. A map of the locations where birds were exposed to air pollution in situ in the studies listed in table 1. Map created using Google Maps.
Download figure:
Standard image High-resolution imageResearchers have documented avian responses to both industrial and urban air pollution by studying birds in air pollution gradients and by comparing the characteristics of birds captured from industrial and urban areas to those from less polluted sites. While some studies do include information about atmospheric contaminants at study sites, many do not quantify exposure or nearby ambient concentrations of specific chemicals. Quantitative measures of pollutants are rarely used to characterize the sensitivity of a response to specific contaminants. Rather, most study results reflect a comparison between birds of the same species from more or less polluted sites. While the statistical significance of these comparative studies indicates that direct exposure to air pollution is affecting birds, additional confounding variables such as habitat degradation and changes in diet may also contribute to the responses observed. Without exposure estimates for individual contaminants, there is insufficient data to quantify air pollution risk to wild bird populations.
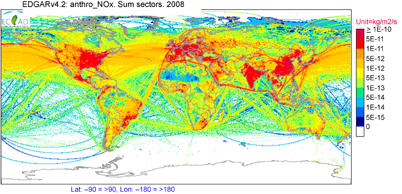
The main challenge in characterizing birds' exposure to specific air pollutants is the availability of ambient air pollution measurements for comparison with ecological and biological outcomes. Epidemiological studies of human health risk from air pollution often rely on centrally located ambient monitors as a proxy for individual exposure (e.g. the network of surface air monitors run by the United States Environmental Protection Agency). These surface monitors could also support analysis of risks to birds, with sufficient information on species habitat and migration patterns. However, most areas of the world do not have ground-based air pollution monitors, and short-term measurements can be difficult, expensive, and of limited relevance to long-term exposure (depending on the duration of the measurements and the variability of the pollutant). Two additional data sources from the atmospheric chemistry community could supplement in situ chemical measurements for the benefit of the ornithology community: chemical transport models (CTMs) and satellite data. Most widely used CTMs provide calculated concentrations of the gas and particulate pollutants discussed in this review, and models have been used to estimate human and vegetation exposure in the absence of in situ measurements. CTMs take information on natural and anthropogenic emissions, with a global example shown in figure 6, and calculate ambient concentrations based on chemical reactions and meteorological transport. Satellite retrievals also offer promise for health- and ecosystem-relevant pollutants, including NO2, CO, and PM, with available global data on a near daily basis going back as far as 1999, depending on the instrument. Laboratory-based risk factors, discussed above, and ambient air pollution concentrations from measurements, models, and/or satellite data could be combined to estimate bird health risk on a local, regional, or global scale.
Figure 6. Map showing total anthropogenic emissions of nitrogen oxides (NOx) in 2008. NOx is emitted by all combustion processes, and thus serves as an indicator of emissions from all fuel types. This map does not show the ambient concentrations of pollutants birds are exposed to, but rather highlights where anthropogenic air pollution would be expected to pose the greatest risk to bird communities. Map created using ECCAD (Emissions of Atmospheric Compounds & Compilation of Ancillary Data), maintained by the GEIA (Global Emissions Initiative), based on data included in the Emission Database for Global Atmospheric Research (EDGAR).
Download figure:
Standard image High-resolution imageWhile potentially informative, such integration of laboratory exposure studies and ambient pollution estimates would have limitations. Rombout et al (1991) point out that results from laboratory studies must be interpreted with caution, as these studies often use inhalation chamber techniques, which require that birds are stationary during exposure. In the wild, birds would have higher metabolic rates, which would potentially magnify the negative effects of air pollution on the respiratory system (Rombout et al 1991, Gorriz et al 1994). Sterner (1993a, 1993b) discusses how restricting birds to cages may result in behavioral changes that are not actually due to elevated concentrations of reactive gases and aerosols. He refers to this as the 'chamber confinement effect.' Finally, Llacuna et al (1993) also point out that the results of laboratory studies examining avian responses to exposure to a single pollutant are important, though not sufficient, in characterizing the impacts of air pollution on avifauna in natural settings, where they are exposed to a combination of atmospheric contaminants of varying concentrations. Some of these same limitations already affect the characterization of air pollution risk to humans, including the challenge of extrapolating laboratory studies to real-world conditions (e.g. toxicological results of rats and other non-human species), and isolating the effects of a single pollutant health risk when exposure occurs simultaneously to multiple pollutants.
While physiological responses to exposure to air pollutants may serve as examples of phenotypic plasticity, this is not discussed in the studies cited in this review. The capacity for both phenotypic plasticity and adaptation in response to increased concentrations of near-surface reactive gases and aerosols could inform which species may face a greater long-term risk from air pollution exposure.
As we improve the characterization of air pollution risk to birds, it is important to consider interaction of the chemicals in the air with other aspects of global environmental change, including shifts in land use and climate change (Lovett et al 2009). For example, urbanization affects both land use and air quality, and warmer temperatures associated with climate change affect air pollution emissions (e.g. smoke from wildfires) and chemical reactions in the atmosphere (e.g. production of ozone) (Jacob and Winner 2009).
Of the roughly 10 000 species of birds known worldwide, only a few have been studied to characterize avian responses to air pollution, and the animals used in laboratory experiments may not be representative of the wild bird species most at risk from air pollution. Future studies should work to identify which species across the globe may be most sensitive to air pollution, as well as any potential differences in response dependent on age and/or sex. Future research on avian responses to air pollution, especially of endangered species, could inform bird conservation programs and improve management of wild bird populations.
Acknowledgments
Support for this project was provided by the National Science Foundation Graduate Research Fellowship Program under Grant No. DGE-1256259, with additional support from the National Aeronautics and Space Administration (NASA) Applied Sciences Program through the Air Quality Applied Sciences Team (AQAST) and Health & Air Quality Applied Sciences Team (HAQAST) initiatives. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the National Science Foundation or NASA. We would like to thank Dr. Benjamin Zuckerberg (Department of Forest and Wildlife Ecology, University of Wisconsin–Madison) for helpful discussions on the topic of this review. We also thank Dr. Alexandra Karambelas (The Earth Institute, Columbia University), Dr. Daegan Miller (Nelson Institute Center for Sustainability and the Global Environment, University of Wisconsin–Madison), Nick Etzel (Department of Biostatistics, University of Washington), Liz Wendt (Medical College of Wisconsin), Becky Reese (Wisconsin Institute for Medical Research, University of Wisconsin–Madison), and anonymous reviewers for taking the time to provide feedback on drafts of this literature review.