Abstract
The tin dioxide nanoparticles (SnO2 NPs) were fabricated via eco-friendly process using Daphne mucronata (D. mucronata) leaves extract as capping and reducing agent. The N2 adsorption/desorption experiment was performed to determine the surface area by Brunauer–Emmett–Teller (BET) method and SBET was found to be 147 m2 g−1. The crystalline nature and lattice parameter was studied by x-ray diffraction (XRD) and calculated crystallite size is 15.63 nm. The surface morphology was examined by scanning electron microscopy (SEM) and the estimated average particle size is 64 nm. The percentage composition and purity of the SnO2 NPs was determined by energy dispersive x-ray (EDX). The raman active modes were identified by using raman spectroscopy while functional groups upon the surface were studied by using fourier transform infrared (FTIR) spectroscopy. The photocatalytic performance of SnO2 NPs was examined against Rhodamine 6G (R6G) and 99.70% rhodamine 6G (R6G) were degraded in 390 min with the degradation rate of 0.0148 per min. The SnO2 NPs were screened against the selected microorganisms and the order of antimicrobial activity is given as; Gram negative bacteria (GNB) > Gram positive bacteria (GPB) > fungi.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
The water pollution is a serious threat to human health and aquatic life all over the world (Fiorenza et al 2018). The large amount of water used in textile industry for various processes released to aquatic environment along with toxic organic dyes (Ciocarlan et al 2018). The products resulting from photochemical oxidation and hydrolysis reactions occurred in wastewater containing organic dyes are harmful to aquatic life. Among these, rhodamine 6G (R6G) is a basic organic dye extensively utilized in acrylic, nylon, silk and wool dyeing (Hameed and El-Khaiary 2008). The long time exposure to R6G can cause severe irritation to eye and respiratory system and also prove fatal to eukaryotic cell mitochondria and its remediation is essential to secure human health, environment and marine life (Senturk et al 2010). Moreover, the development of multi-drug resistant (MDR) microbial strains is also growing social problem arose due to the improper and extensive use of broad spectrum antibiotics (Meymandi et al 2010). The widespread use of antibiotics in foodstuff, animal husbandry and domestic cleaners led to the development of antibiotic resistant microbial strain (Chaisatit et al 2010). Many bacterial species like Salmonella, Escherichia coli, Staphylococcus aureus, Enterococcus and Pseudomonas species are highly resistant to the common antibiotics (Zare et al 2017). Thus, the presence of toxic organic dyes and MDR microbes are the most serious thread toward human health and aquatic life. Thus, these problems should be solve to protect the biotic components of ecosystem.
To meet these challenges, the current research was planned to prepare nanosized SnO2 for photocatalytic and antimicrobial applications. The SnO2 is an important n-type semiconductor having band gap of 3.6 eV, exhibit unique size and shape dependent properties including optical, electronic, electrochemical and catalytic properties (Tan et al 2008; Amininezhad et al 2015). The SnO2 NPs is widely used as sensors, conducting electrode, rechargeable batteries, optical and electronic devices (Hu et al 2002, Liu et al 2005, Ho et al 2009, Yeow et al 2009). Numerous procedures have been reported for the synthesis of SnO2 NPs including sol-gel, hydrothermal, co-precipitation, solvothermal, chemical vapor deposition and carbothermal reduction process (Leite et al 2002, Fujihara et al 2004, Liu et al 2005, Qiao et al 2014, Bhagwat et al 2015, Nadaf and Venkatesh 2016). However, the use of plant materials for the synthesis of nanomaterials is more advantageous due to its easy handling, environment friendly and economical nature (Haq et al 2016).
The D. mucronata (green source) used in the present work for the environment-friendly preparation of SnO2 NPs, belongs to the family Thymelaeaceae and its leaves are of medicinal importance and used for the treatment of anti-leukemia, anti-gout and anti-inflammatory (Zaidi et al ). The surface and structural properties of the prepared SnO2 NPs were studied by BET method utilizing N2 sorption/desorption data, SEM, XRD, EDX, FTIR and raman spectroscopies. The photocatalytic performance of the SnO2 nanoparticles was tested against the R6G in aqueous solution. The SnO2 NPs were also screened for the antimicrobial activity against Bacillus subtilis (B. subtilis), Staphylococcus aureus (S. aureus) as GPB, Escherichia coli (E. coli), Pseudomonas aeruginosa (P. aeruginosa) as GNB, Candida albicans (C. albicans) and Aspergillus niger (A. niger) as fungi.
2. Materials and methods
2.1. Materials
The analytical grade chemicals; tin (IV) chloride pentahydrate (SnCl4.5H2O), rhodamine 6G, ethanol (C2H5OH) and tryptic soy agar were acquired from Sigma Aldrich and utilized without further purification. All the solutions/suspension were prepared in deionized water. The fresh leaves of D. mucronata were collected from the Anak Buner and taxonomically identified by the department of Botany.
2.2. Formation of D. mucronata leaves Extract
The D. mucronata leaves were thoroughly washed with tape water to remove dust particles followed by deionized water and were dried in shade at room temperature. For the preparation of extract, 20 g of the clean and dry leaves were added into an air tight jar containing 500 ml boiled deionized water for 1 h. The mixture was then allowed to cool at room temperature and filtered followed by centrifugation at 5000 rpm for 15 min The upper layer of yellowish green extract was stored at 4 °C for further experiment.
2.3. Fabrication of SnO2 NPs
The desired SnO2 NPs were synthesized by mixing 60 ml of SnCl4.5H2O (0.003 M) with 20 ml of D. mucronata leaves extract with continuous heating (55 °C) and stirring (500 rpm) and the greenish gel obtained after 40 min was aged for 24 h. The aged gel was washed thrice with hot deionized water by adding hot deionized water and stirred (2 min) and then allowed for sufficient time to settle down. The gel was filtered and washed again with ethanol and dried in oven at 100 °C for 6 h. The obtained colorless crystals were crushed into fine powder and kept in polyethylene bottle.
2.4. Characterization
The N2 adsorption-desorption experiment was performed by micromeritics model GeminiVII2390i and the surface area was estimated by Brunauer–Emmett–Teller (BET) method. The crystal properties was studied by x-rays diffractometer model Panalytical X-Pert Pro and the crystallite size was calculated by Debye–Scherrer equation. The elemental composition and purity was determined by EDX model INCA 200 (UK) coupled with SEM microanalyzer model JEOL 5910 (Japan) to identify the morphology of the SnO2 particles. The Raman analysis was performed in the of 100–1000 cm−1 raman shift using Micro Horiba (Xplora Plus Microsscope) equipped with a 532 nm laser. The FTIR analysis was carried out via Nicolet 6700 (USA) using KBr pellets in the range of 4000–400 cm−1.
2.5. Photocatalytic activity
The photocatalytic degradation experiment of R6G was performed in a double layer borosilicate reactor in the presence of SnO2 NPs and simulated solar light source (US-800 (250 W). For the experiment, 20 mg of SnO2 NPs (0.4 g L−1) was added to reaction vessel, covered with aluminum foil containing 50 ml of R6G solution (15 ppm) and was agitated in dark for 30 min to attend the adsorption-desorption equilibrium. The reaction mixture was than illuminated with simulated solar light and 3 ml of the sample was analyzed after a specific intervals of time (i.e. 5, 15, 30, 50, 75, 105, 140, 180, 225, 275, 330 and 390 min). The reduction in the absorbance maxima at 526 nm with increasing irradiation time was examined through double beam spectrophotometer model Thermo Spectronic UV 500.
2.6. Antimicrobial activity
The antimicrobial efficacy of the SnO2 NPs was examined against the selected microbes by agar well diffusion method. The microbial culture was streaked over pre-incubated tryptic soy agar nutrients plates and the wells were bored in the nutrient media with sterile borer. For suspension preparation, 3 and 5 mg of SnO2 NPs was ultrasonically dispersed 5 ml of deionized water and the wells were loaded independently with 40 and 60 μl of these suspensions to observe the effect of concentration and volume of the SnO2 NPs on the antimicrobial activity. The plates containing the bacteria inoculum were incubated at 37 °C for 24 h whereas those having fungal species were incubated at 25 °C for 4 days. The antimicrobial activity of SnO2 NPs was estimated from the zones of inhibition measured in millimeters (mm) at each concentration and volume.
3. Results and discussion
3.1. Physicochemical study
The type II N2 adsorption/desorption curve (figure 1) describe the nonporous nature of SnO2 NPs according IUAPC classification (Thommes et al 2015) and SBET was found to be 147 m2 g−1, which higher than the previously reported data (Haq et al 2019a). The pore size and pore volume estimated from the desorption curve are 15.64 Ǻ and 0.020 c.c./g respectively. The XRD pattern of the SnO2 NPs presented in figure 2, displays characteristic Bragg's reflections at 2θ position with corresponding hkl values are 26.88(112), 33.80(006), 44.59(204), 51.78(130), 57.63(119) and 64.82(233). All these diffraction bands are found similar with the peaks listed in JCPDS card 01-077-0450 attributed to the tetragonal crystal system of SnO2 NPs. The crystallite size calculated by Debye Scherer's equation is 15.63 nm, whereas, 1.16% imperfection was found in the crystal (Haq et al 2016).
Figure 1. N2 adsorption-desorption isotherm of SnO2 NPs.
Download figure:
Standard image High-resolution imageFigure 2. X-ray diffractogram of SnO2 NPs.
Download figure:
Standard image High-resolution imageThe EDX spectrum posted in figure 3, possess an intense band at 3.5 keV along with a less intense signal at 0.5 keV are due to the Sn and O respectively. The presence of C and N in the sample might be due to the use of plant material used in the synthetic process. The weight percentage of Sn, O, N and C estimated from the EDX analysis are 62.68, 33.90, 1.25 and 2.17 respectively. The SEM image given as inset in figure 3, indicates that the particles are interconnected with each other, however some particles with visible boundaries were also observed, which exhibit different shape and size. The particles sizes estimated from SEM micrograph are range between 57 to 72 nm with average particle size of 64 nm, which is almost four time larger than the crystallite size.
Figure 3. EDX spectrum (inset: SEM) of SnO2 NPs.
Download figure:
Standard image High-resolution imageThe raman spectrum of SnO2 NPs (figure 4) possess three bands at 347.30, 576.17 and 643 cm−1. The peak at 347.30 cm−1 is due to the surface area of nanosized SnO2 with tetragonal geometry (Cheng et al 2004). The band at 576.17 cm−1 was assigned to amorphous content of the Sn(OH)4 (Ristić et al 2002), whereas the raman mode at 643 cm−1 described to A1g assigned to the expansion and contraction of Sn-O band. The FTIR spectrum of SnO2 NPs shown as inset in figure 4, possess a broad band in range of 3538–2925 cm−1 and a sharp band at 1619.65 cm−1 are due to the stretching and bending vibration of O–H moiety respectively (Rehman et al 2019). The set of peaks in the range of 1550 to 890 cm−1 is due to the vibration of hydroxyl tin bonds (Haq et al 2019a). The bands at 662.90 and 571.85 cm−1 corresponding to the vibrations of O–Sn–O and terminal Sn–OH moiety respectively (Haq et al 2019b).
Figure 4. Raman spectrum (inset: FTIR spectrum) of SnO2 NPs.
Download figure:
Standard image High-resolution imageThe exact mechanism of the green synthetic route is not known, however, it is believed that the phytochemicals act is reducing agents as well as capping agent. The formation of metal oxide nanoparticles consist of reduction of ions, nucleation, cluster formation, growth and oxidation of metal nanoparticles (Jain and Mehata 2017). Each of these stages depends upon the nature of reducing agent, its concentration, precursor salt concentration, temperature and pH. The main phytochemicals present in D. mucronata are coumarins, carboxylic acid, flavonoids, triterpenoids, lignin and cumarinolignans, which are responsible for bioreduction of nanoparticles (Zaidi et al 2015). The hydroxyl group present in the phytochemicals are responsible for the reduction of metal ions, which are capped with phytochemicals. However, it was assume earlier on the basis of DFT analysis that the hydroxyl group present in catechol moiety of flavonoid have low dissociation energy as compared to the other hydroxyl groups of flavonoid and phytochemicals (Singh et al 2018). The metal ions capped with phytochemicals when dried in air convert into metal oxides (Imran Din and Rani 2016).
3.2. Photocatalytic study
The photocatalytic efficacy of SnO2 NPs was examined against the R6G in aqueous solution by a solid/liquid ratio of 0.4 g l−1 and the absorbance maxima at 526 nm was recorded. After 30 min equilibrium time, the R6G solution was expose to simulated solar light source and the degradation was visually observed by the gradual changing of reddish purple color to colorless. The characteristic absorbance maxima at 526 nm was gradually decrease with increasing the light illumination time as given in figure 5(a). The equation (1) was used to calculate percentage degradation and almost all the R6G (99.70%) was degraded in 390 min The stability of the R6G was also examined under the simulated solar light in the absence of SnO2 NPs for 390 min and only 1.39% R6G was degraded. The photocatalytic efficiency of SnO2 NPs was increases with increasing the irradiation time under simulated solar light and ultimately percentage degradation also increases. The experimental data was fitted well into the Langmuir-Hinshelwood kinetics model (equation (2)), where k is degradation rate constant, Co is initial concentration while C is the concentration of R6G at time (t). The plot ln(Co/C) versus time (t) shows linear relationship suggesting that the photocatalytic degradation follow pseudo first order reaction. The degradation rate constant (k) determined from the plot c (figure 5(c)) proposed that the 0.0148 amount of R6G was degraded in 1 min Similar observation was experienced previously during the photocatalytic degradation of methylene blue in the presence of nano-sized SnO2 (Fu et al 2015, Elango and Roopan 2016).
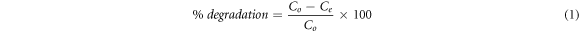
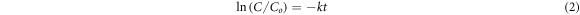
Figure 5. (a) Degradation profile, (b) % degradation, (c) Kinetic isotherm and (d) schematic mechanism of the photocatalytic activity.
Download figure:
Standard image High-resolution imageThe photocatalytic phenomena occur when the incident light beam fall on the surface of catalyst having equivalent or higher energy than the band gap of the catalyst, the outer electron get excited into conduction band. As a result, positive hole (h+) generated in the valance shell, which reacts with surface hydroxyl group or water molecules to produce hydroxyl radical (·OH), which act as strong oxidizing agent. The electron promoted to conduction band trapped by the physisorbed oxygen to produce superoxide anion radicals (·O−), which is strong reducing agent and additional source of ·OH radical production. The interaction of superoxide anion radicals (·O−) with hydrogen (+H) also led to the formation of hydroxyl radicals. The CO2 and H2O molecules were produced from the intermediate simple organic compounds, formed during the reaction of ·OH and R6G (Fu et al 2015).
3.3. Antimicrobial study
The antimicrobial activity of SnO2 NPs was examined against the selected microorganisms and the experimental photographs are posted in figures 6 (a) to (f). The zones of inhibition measured in millimeter (mm) are tabulated in table 1, shows that the GPB indicates greater resistance against SnO2 NPs as compare to GNB and fungi. The SnO2 NPs shows antifungal activity only at higher concentration (5 mg 5 ml−1) against both fungi. The activity was seen to be enhanced with increasing the concentration and volume of the suspension in the wells. The results also shows that the activity of SnO2 NPs is less than the standard drug while solvent was found inactivity against the tested microorganisms. The difference in the antimicrobial activity of SnO2 NPs was due to the difference in the surface electrostatic force, composition and structure of the cell wall (Prameela Devi et al 2013). The surface of GPB has weak negative charge due to the presence of phosphate moiety in teichoic acid, whereas, the strong negative charge present at the surface of the GNB is due to the presence of phospholipids and lipopolysaccharides. The enhanced activity against GNB was due to the strong interaction of Sn2+ with strong negative charge as compared to GPB and fungi. Thus larger number of Sn2+ accumulate on the surface of against GNB causing cell rupturing and penetrate inside the cells easily. The difference in the activity may also due to the presence of thick and rigid peptidoglycans layer in the cell wall of GPB as compared to GNB, which offer additional strength and become more resistant against Sn2+ ions (Amininezhad et al 2015). The lower antifungal activity of SnO2 NPs is due to the weak surface electrostatic forces and the composition of the outer membrane. The fungus cell membrane is surrounded by more complicated cell wall, thus penetration of incoming therapeutic agent is more difficult (Haq et al 2018a). The antimicrobial activity of SnO2 NPs may also be due to the presence of metal cation, superoxide radical, hydroxyl radical and also due to the release of oxygen on the surface of SnO2 NPs (Shah et al 2019). The antimicrobial action of the SnO2 NPs is due to the presence of superoxide and hydroxyl radicals which led to the formation of H2O2, which penetrate inside the cell and disturb the composition of cytoplasm, ultimately the microorganism unable to survive (Haq et al 2018b, Shah et al 2019).
Figure 6. Antibacterial activity of SnO2 NPs. S = 3 mg/5 ml, SL = 5 mg/5 ml, NC = −ve control (solvent), PC = positive control (Levofloxacin for bacteria and Fluconazole for fungi), 40 and 60 = volume of solution in microliter (μl).
Download figure:
Standard image High-resolution imageTable 1. The inhibition zones measured in millimeter as the antimicrobial activity of SnO2 NPs.
| Samples | Gram positive bacteria | Gram negative bacteria | Fungi | |||
|---|---|---|---|---|---|---|
| B. Subtilis | S. aureus | E. coli | P. Aeruginosa | C. Albicans | A. Niger | |
| NC | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| S-40 | 0.00 | 0.00 | 1.9 | 1.8 | 0.00 | 0.00 |
| S-60 | 4.0 | 4.5 | 3.3 | 3.3 | 2.0 | 2.2 |
| SL-40 | 4.4 | 4.8 | 4.6 | 4.6 | 3.4 | 3.1 |
| SL-60 | 6.8 | 5.6 | 6.9 | 6.9 | 4.9 | 4.6 |
| PC | 11.2 | 9.8 | 8.0 | 8.5 | 6.1 | 6.3 |
S = 3 mg/5 ml, SL = 5 mg/5 ml, NC = −ve control (solvent), PC = positive control, 40 and 60 = volume of solution in microliter (μl).
4. Conclusion
The nonporous nanocrystalline SnO2 NPs with high surface area was successfully prepared by using D. mucronata leaves extract as reducing and capping agent. The 99.70% of R6G was degraded in the presence of SnO2 nanocatalyst in 390 min with the degradation rate of 1.48 × 10−2 min−1. The photocatalytic performance of SnO2 NPs was increased with increasing irradiation time under simulated sun light source. The antimicrobial activity of SnO2 NPs was high against GNB as compared to against GPB and fungi. The activity was seen to be increased with increasing the concentration and volume of the SnO2 suspension in the wells. The difference in the activity was due to the change in the surface charge and cell wall composition of the microorganisms.
Acknowledgments
Dr Sirajul Haq is highly thankful to Laboratory of Adsorption and Catalysis (LADCA), Department of Chemistry, University of Antwerp, Belgium for providing space and research facilities during doctorate study.








