Abstract
It is a controversial issue whether the electric current pulse (ECP) has an inoculation effect on the solidification process. Two different high chromium cast irons were modified with the ECP above the freezing point temperature to investigate this issue. According to the statistical results of the number and size of primary phase grains, it was found that the ECP did not have an inoculation effect on the hypoeutectic high chromium cast irons (HOHCCI) whose primary phase was austenite. However, it had a significant inoculation effect on the hypereutectic high chromium cast irons (HEHCCI) whose primary phase was M7C3 carbides. The result indicates that the response of different primary phases to the ECP inoculation effect was different. The cause of the phenomenon is further analyzed by simulating the diffusion of atoms in the melt and comparing the binding forces of atomic clusters. The analysis shows that although the ECP can increase the number of atomic clusters (composition close to the primary phase) in the melt, the number of clusters retained to the nucleation temperature after the ECP removal is different because the binding forces of clusters in two melt is different.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Since the electric pulse current (ECP) was found to be able to refine the solidified microstructure [1], most scholars have applied the ECP during the nucleation stage, or the growing stage of the grains to research the influences of the ECP on the microstructure refinement and have put forward some theories to explain this phenomenon. Nakada et al [1, 2] pointed out that the pinch force caused by the ECP may change at various points in the Sn-15Pb sample, which generates shear stresses to break dendrites into globular grains. The voltage of the ECP has an essential effect on the modification effect of the sample structure. If the fraction of the solid phase in the melt, grain refinement requires a higher voltage. Conrad [2, 3] et al studied the effect of the ECP on the microstructure of 60Sn40Pb and 63Sn37Pb alloys. The ECP reduces the size of the eutectic colony. The authors pointed out that the main reason for refining the microstructure is that the ECP changes the free energy or interface energy, rather than the skin effect or pinch effect of the ECP. The study by Rabiger et al [4] showed that there was no significant difference between the effects of direct current and the rectangular ECP on the Al-7Si microstructure when the valid value of the current was the same. The author attributed the microstructure refinement to the forced convection in the melt. Gao et al [5] believed that the grain refinement is caused by the uniform nucleation inside the melt when the ECP was applied to the ZA27 melt that begun to solidify.
To give full play to the effect of the ECP in the solidification, some scholars extend the temperature range of pulse application to a superheated melt. However, they get different phenomena and conclusions. Zhai et al [6, 7] held that the ECP could not refine pure Al grain when the ECP was applied above the freezing point temperature. However, Wang and Qi et al [8, 9] believed that the ECP could refine grains of the pure aluminum or the Al-5%Cu alloy because the ECP could change the size and relative quantity of atomic clusters when the ECP was applied above the freezing point temperature. Geng et al studies [10, 11] had shown that the size of primary carbides significantly reduced when the ECP was applied to overheated melt of the hypereutectic high chromium cast iron.
Qin et al [12, 13] pointed out that the free energy changes of the two phases are different when current flows through two phases because the conductivity of the two phases is different. As all know, there is an additional term for the free energy difference. There are many clusters in the melt that contain different numbers of atoms [14–16]. Although the structure of the cluster is different from that of the solid crystal, it already has a regular structure compared with the disordered liquid phase [16, 17]. In this view, atomic clusters and disordered liquid phase has different conductivity. Therefore, Geng et al [11] believed that the difference in the additional free energy between the atomic clusters and the disordered liquid phase would change the number of atomic clusters when the ECP was applied on a melt. The corrected equilibrium number (Nn) of atomic clusters can be given by equation (1) when the ECP was applied on a melt [11]:
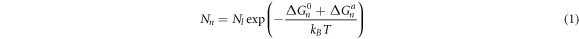
where  is the total number of atoms in the melt;
is the total number of atoms in the melt;  is Boltzmann constant; T is the melt temperature;
is Boltzmann constant; T is the melt temperature;  is the difference in free energy of an atom in the cluster and the disordered liquid phase when the ECP is not applied to melt.
is the difference in free energy of an atom in the cluster and the disordered liquid phase when the ECP is not applied to melt.  is the additional free energy difference caused by the ECP.
is the additional free energy difference caused by the ECP.  is negative because the conductivity of the cluster is higher than that of the disordered liquid phase, which leads to an increase in the equilibrium number of atomic clusters. The increase in the number of atomic clusters increases the nucleation rate. The nucleation rate
is negative because the conductivity of the cluster is higher than that of the disordered liquid phase, which leads to an increase in the equilibrium number of atomic clusters. The increase in the number of atomic clusters increases the nucleation rate. The nucleation rate  be given by an expression [17]:
be given by an expression [17]:
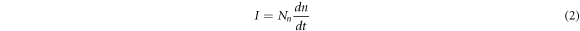
where,  is the atomic adsorption rate. The increase in the nucleation rate leads to an increase in the number of nucleation during the subsequent solidification process, and the grains are refined.
is the atomic adsorption rate. The increase in the nucleation rate leads to an increase in the number of nucleation during the subsequent solidification process, and the grains are refined.
We speculate that whether the ECP has an inoculation effect is not only related to the ECP treatment process but also related to the nature of the primary phase in the material. Two high chromium cast irons with different compositions were selected in this work to verify the speculation. The high chromium cast iron is widely used as a wear-resistant material [18–21]. It is commonly used in mining, metallurgy, electricity, and other fields [19, 20, 22–24]. Scholars often enhance material toughness and ultimately increase the service life of high chromium cast iron by adding alloy elements [24–29] or special casting [23, 30, 31]. The ECP modification still provides a new idea for improving the microstructure and performance of the high chromium cast iron, and the ECP modification is highly efficient and clean. Therefore, this work can not only further supplement the ECP machining theory but also provide an efficient and environmentally friendly process for improving the microstructure of high chromium cast iron.
2. Materials and methods
Two high chromium cast irons were prepared. They are hypoeutectic high chromium cast irons (HOHCCI) and hypereutectic high chromium cast irons (HEHCCI). The detailed composition is shown in table 1. The simultaneous thermal analyzer (NETZSCH STA 449F3, NETZSCH, Selb, Germany) was used to analyze liquidus temperature and primary phase precipitation temperature, and the rate of temperature rise and fall is 10 °C per minute. For the HOHCCI, the liquidus temperature is about 1335 °C, and the primary phase start precipitation temperature is 1323 °C; For HEHCCI, 1337 °C and 1327 °C for HEHCCI.
Table 1. Composition of high chromium cast irons (wt%).
| Sample no. | Material | Cr | C | Si | Fe |
|---|---|---|---|---|---|
| A1–A5 | HOHCCI | 14.77 | 2.82 | 0.972 | balance |
| B1–B5 | HEHCCI | 20.76 | 3.95 | 1.31 | balance |
Figure 1 shows the schematic sketch of the experimental setup. The specific treatment process is as follows. First, the rods of Φ18 mm * 150 mm were cut from a block of high chromium cast iron using wire cutting technology. Second, a HOHCCI or HEHCCI rod was put into the alumina tube, and the two ends of the alumina tube are sealed with the Nichrome aluminum rod electrode and the sand bonded by sodium silicate. Third, put the packaged sample into the tube muffle furnace (YFK60-600/160, Shanghai Yifeng Electric Furnace Company, Shanghai, China). The sample was heated from room temperature to 1000 °C at a heating rate of 10 °C per minute. For reducing the internal temperature difference of the sample, the sample was heated at a heating rate of 5 °C per minute from 1000 °C to 1376 °C for HOHCCI and from 1000 °C to 1380 °C for HEHCCI. During the period, the sample melted. Fourth, the melt was soaked at the highest temperature for 10 min to make the temperature and composition of the melt uniform. At last, the sample was cooled at the cooling rate of about 10 °C per minute in the furnace, and the ECP was introduced into the melt during cooling. In the whole process of heating and cooling, a platinum-rhodium thermocouple is used to detect and control the melt temperature. See figure 2 and table 2 for a clearer understanding of the timing of the electric current pulse application.
Figure 1. Schematic sketch of experimental setup. Reproduced from [11]. © IOP Publishing Ltd. All rights reserved.
Download figure:
Standard image High-resolution imageFigure 2. The ECP modified processing for different samples. (a) HOHCCI, (b) HOHCCI.
Download figure:
Standard image High-resolution imageTable 2. Temperature range of pulse current processing.
| TR 1 | SD 2 | TR 1 | SD 2 | ||
|---|---|---|---|---|---|
| A1 | Without ECP | Without ECP | B1 | Without ECP | Without ECP |
| A2 | 1376–1353 °C | 30 °C | B2 | 1380–1357 °C | 30 °C |
| A3 | 1376–1338 °C | 15 °C | B3 | 1380–1342 °C | 15 °C |
| A4 | 1376–1333 °C | 10 °C | B4 | 1380–1338 °C | 10 °C |
| A5 | 1376–1328 °C | 5 °C | B5 | 1380–1332 °C | 5 °C |
1 TR is the temperature range of the electric current pulse application. 2 SD is the melt superheat degree when pulse current is removed.
Previous work [11] had shown that the ECP had the most obvious incubation effect on primary carbides when the ECP parameter was 500 A (196.6 A cm−2), 10 μs, and 45 Hz. Therefore, the ECP parameter used was 500 A, 10 μs, 45 Hz, and remains unchanged in this work. Only change the material and timing of pulse current modification to explore the influence of material and timing on the ECP inoculation effect.
The samples were corroded in a 5% FeCl3 aqueous solution for 5 s. An optical microscope is used to analyze the microstructure of the sample. Software Image-J was used to calculate the size and number of primary phases. For the HOHCCI, the primary phase is austenite with dendritic shapes. It is difficult to obtain the exact number of austenite dendrite. The number and size of the dendritic grains are approximately measured by measuring the distance of the secondary arm and the equivalent diameter of all dendritic arms (the primary and secondary arms are considered as grains). The statistics of the secondary arm spacing are derived from 50 dendritic grains in the 5–10 metallographic pictures. The statistics of the equivalent diameter and the shape factor derived from 5 metallographic pictures. The size corresponding to each metallographic picture is 3.1 mm * 3.1 mm. For the HEHCCI, the primary phase is the M7C3 carbides [32]. The transverse section width of the carbide can better represent the size of the primary carbide [11]. The size and number of primary carbides are counted in the three metallographic diagrams. It should be noted that sometimes primary carbides and eutectic carbides are not easy to distinguish in the HEHCCI. The transverse sectional width less than 25 μm are considered eutectic carbides and are not included in statistics, because the average width of the largest eutectic carbide is 25 μm, which were measured by cutting the line method. The element content of primary austenite and carbides was analyzed with an electron probe micro-analyzer (JXA8230), and the test current was 20 A; the electron beam spot is less than 1 μm.
3. Results
3.1. Influence of the ECP on the size of primary austenite
The microstructure of the A1–A5 samples was shown in figure 3. It is easy to see from figure 3 that the microstructures are very similar. The primary austenite is dendritic, and their shapes and sizes are almost the same. Figure 4 shows the statistical data of primary austenite grains. As all know, the distribution of the metal grain size conforms to the logarithmic normal distribution. The results of the statistics are also following the logarithmic normal distribution, which indicates that the statistics are representative. Figures 4(a)–(c) show that even if the timing of the ECP treatment is different, the distribution of the secondary dendrite arm size, the equivalent dendrite diameter, and the shape factor are almost the same. Figure 4(d) more clearly shows that the average value of the grain density, secondary dendrite arm size, equivalent dendrite diameter, and the shape factor have not changed significantly after the ECP treatment to the melt. It shows that the pulse current has no obvious effect on the nucleation and growth of austenite. The ECP did not have an inoculation effect on the primary austenite.
Figure 3. Microstructure of the HOHCCI samples. (a) A1; (b) A2; (c) A3; (d) A4; (e) A5. The dark area is the primary austenite. The dark and light stripes are eutectic; the dark stripes are eutectic austenite or austenitic transformation products; the light stripes are eutectic carbides.
Download figure:
Standard image High-resolution imageFigure 4. Statistical diagrams of primary austenite grains. (a)–(c) are respectively distribution diagrams of the secondary arm spacing, the equivalent diameter and shape factor; the curves are fitted lognormal distribution curves. (d) the trend of change in the corresponding mean.
Download figure:
Standard image High-resolution image3.2. Influence of the ECP on the size of primary carbides
The microstructure of the B1–B5 was shown in figure 5. The grain size distribution also basically conforms to the logarithmic normal distribution, and the number distribution functions of the five samples differ greatly, see figure 6(a). The mean and mode of sample B4 and B5 were significantly lower than those of sample B1–B3. Compared with the sample B1, the primary carbide size of the sample B2 (the ECP was removed when the superheat degree was 30 °C) is almost unchanged. The primary carbides were significantly refined for the sample B4 or B5 (the ECP was removed when the superheat degree was 10 °C or 5 °C). The average grain size was reduced from 47.8 μm to 33.9 μm, with a reduction of about 30%. The average grain density changed from 932/cm−2 to 1782/cm−2, with an increase of about 90%. See figure 6(b) for details. The result indicates that there was a significant inoculation effect on primary carbides when the melt was modified with the ECP at a low superheat degree.
Figure 5. The microstructure of the HEHCCI samples. (a) B1; (b) B2; (c) B3; (d) B4; (e) B5. The light area is the primary carbides. The dark-and-light stripes are eutectic; the dark stripes are eutectic austenite or austenitic transformation products; the light stripes are eutectic carbides.
Download figure:
Standard image High-resolution imageFigure 6. Statistical diagrams of the primary carbides. (a) Distribution diagrams of the size of the primary carbides; the curves are fitted lognormal distribution curves. (b) The trend of change in the average grain density and size of the primary carbides.
Download figure:
Standard image High-resolution image4. Discussion
The ECP can increase the number of the atomic clusters and leaves the superheated melt in a non-equilibrium state [11]. Although we cannot currently observe how the large-sized clusters further evolve after the ECP was removed, the number of which is more than the equilibrium number, we can make a reasonable inference that the excessive clusters would inevitably disappear and bring the melt back to thermodynamic equilibrium state according to the thermodynamic principle. The influence of ECP on the melt disappears when the excessive atomic clusters disappear completely. However, the test results show that the ECP had an inoculation effect on primary carbides when the temperature close to the precipitation temperature of primary carbides. The influences of the ECP on the melt completely disappeared need to be considered from two aspects. The first is the disintegration of the excessive clusters. The second is that more solvent or solute atoms in the clusters need to diffuse uniformly after the clusters disintegrate. The composition of the clusters in the low superheat melt should be similar to that of the primary phase, and such clusters are called primary-phase-like atomic clusters in the following. The composition of primary-phase-like clusters is different from the disordered liquid phase. The time required for each of these two processes is of great concern to us.
The time required for the cluster to disintegrate due to energy fluctuations in the melt is currently inconclusive, and the corresponding calculation and simulation are challenging, but analyze diffusion with Fick's law is relatively easy. We first assume that the cluster disintegrates immediately after removing the ECP, and build a model to simulate the time taken for diffusion. The solute diffusion is related to the concentration gradient but independent of the absolute concentration. Therefore, the actual diffusion process can be simplified to a model in which the initial mass is  in the center area of the cluster, and the concentration in the cluster region Δc is
in the center area of the cluster, and the concentration in the cluster region Δc is  and the concentration Δc is 0 g · cm−3 in other regions. Where,
and the concentration Δc is 0 g · cm−3 in other regions. Where, 
 is the initial concentration of solutes in the cluster,
is the initial concentration of solutes in the cluster,  is the initial concentration of solutes in the molt,
is the initial concentration of solutes in the molt,  is the cluster volume. The approximate size of the cluster takes the critical nucleus volume of pure iron in this work, and the value is 6.705 * 10−21cm3 (the required thermodynamic parameters for calculation are shown in table 3.). Besides, the clusters which generally contain hundreds of atoms are so small that the cluster is regarded as a particle (ignoring the radius of the cluster) to simplify the diffusion equation in the work. Figure 7 is a schematic of element diffusion.
is the cluster volume. The approximate size of the cluster takes the critical nucleus volume of pure iron in this work, and the value is 6.705 * 10−21cm3 (the required thermodynamic parameters for calculation are shown in table 3.). Besides, the clusters which generally contain hundreds of atoms are so small that the cluster is regarded as a particle (ignoring the radius of the cluster) to simplify the diffusion equation in the work. Figure 7 is a schematic of element diffusion.
Table 3. Thermodynamic parameters.
| Melting latent heat ΔHm/J * mol−1 | Molar volume Vmol/m3 | Interfacial energy σ/J * m−2 | Melting point/ Undercooling Tm/ΔT |
|---|---|---|---|
| 1.52 * 103 | 8.72 * 10−7 | 0.204 | 0.2 |
Figure 7. Schematic of element diffusion.
Download figure:
Standard image High-resolution imageThe type of diffusion is non-steady-state spherically symmetric diffusion, and its diffusion equation is as following [33]:
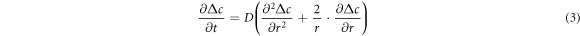
where  is the difference between the solute concentration in the melt after diffusion and the initial solute concentration of the melt, g · cm−3;
is the difference between the solute concentration in the melt after diffusion and the initial solute concentration of the melt, g · cm−3;  is the distance from the center of the cluster, cm.
is the distance from the center of the cluster, cm.  is time, s;
is time, s;  is the diffusion coefficient of the solute in the melt, cm2 · s−1. The Gaussian solution to equation (3) is given in equation (4):
is the diffusion coefficient of the solute in the melt, cm2 · s−1. The Gaussian solution to equation (3) is given in equation (4):
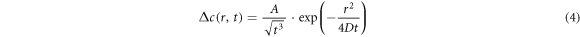
where A is the parameter to be determined, equation (4) should satisfy the condition of equation (5):
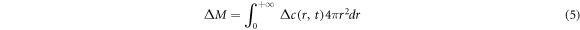
Equation (4) was brought into equation (5), and A can be found as equation (6). Equation (6) was brought into equation (4) to get the concentration function as equation (7):
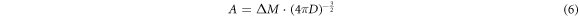
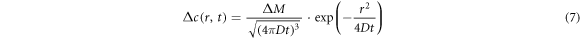
The element concentration in melt and the primary phase cluster required for the calculation of  and the
and the  values are shown in table 4. The diffusion coefficients of elements in the melt are shown in table 5.
values are shown in table 4. The diffusion coefficients of elements in the melt are shown in table 5.
Table 4. The element concentration in melt and cluster and the  values.
values.
| In melt (g cm−3) | In primary phase cluster (g cm−3) | ΔM values (10−20 g) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Cr | Fe | C | Cr | Fe | C | ΔMCr | ΔMFe | ΔMC | |
| HOHCCI | 1.14 | 6.27 | 0.22 | 0.92 | 6.59 | 0.18 | −0.148 | 0.215 | −0.022 |
| HEHCCI | 1.60 | 5.70 | 0.30 | 3.58 | 2.59 | 0.68 | 1.326 | −2.080 | 0.253 |
Table 5. Diffusion coefficient of elements in melt.
| Elements | Cr | Fe | C |
|---|---|---|---|
| Diffusion coefficient D (cm2 · s−1) | 4.3 × 10−5 | 4.64 × 10−5 | 28 × 10−5 |
Since the size of the cluster is neglected in establishing the diffusion model, the calculated results can be close to the real value only if t is higher than ω2 · (2D)−1. The ω is cluster diameter, and we take t as 1 ms to 2 ms and r as 0 μm to 10 μm for the simulation calculation. The Octave software was used to perform simulation calculation of equation (7). The results are shown in figure 8.
Figure 8. Simulation results of diffusion. (a), (c) and (e) are the diffusion results of Cr, Fe, and C in HOHCCI, respectively; (b), (d), and (f) is the diffusion results of Cr, Fe, and C in HOHCCI, respectively. The Δc in (a), (d) and (e) is negative, which means the concentration of Cr and C in a cluster is lower than in the melt for the HOHCCI, and the concentration of Fe in a cluster is lower than in the melt for the HEHCCI.
Download figure:
Standard image High-resolution imageFor the HOHCCI, it can be seen from figures 8(a), (c) and (e) that the difference between the concentration of Cr, Fe and C in center of the original cluster positions and the other positions of the melt is respectively less than 4 * 10−10 g cm−3, 5 * 10−10 g cm−3, and 4 * 10−12 g cm−3 within 1 ms, if the primary-austenite-like atomic clusters are completely disintegrated. Compared with the 0.22 g cm−3, 0.32 g cm−3, and 0.04 g cm−3 before the disintegration of the primary-phase-like atomic clusters, the concentration difference at the moment could be ignored. Therefore, it could be considered that the solute has spread evenly at 1 ms after the disintegration of the atomic clusters. It should be pointed out that the concentration difference of the C after diffusion is lower; The diffusion distance is longer. Because the diffusion rate of C is higher than that of Cr and Fe, as shown in figure 8(e). For HEHCCI, figures 8(b), (d), and (f) shows that the concentration difference of Cr, Fe and C is respectively less than 3.5 * 10−9 g cm−3, 5.0 * 10−9 g cm−3 and 4.0 * 10−11 g cm−3 (center of the original cluster positions). Them is much smaller than the 1.98 g cm−3, 3.11 g cm−3, and 0.38 g cm−3 (the other positions of the melt) so that it can be considered that there is no concentration difference. The phenomenon indicates that the solute could diffuse uniformly in a short time, and the melt returned to the state before the ECP treatment if the excessive primary-phase-like clusters disintegrate immediately after removing the ECP. In other words, the effects of the ECP disappear in a short time, if the excessive primary-phase-like clusters disintegrate immediately after removing the ECP.
However, the primary carbides in HEHCCI were significantly refined when the ECP was removed at 10 °C or 5 °C superheat degree. Therefore, it indicates that the excessive clusters in the HEHCCI melt did not disintegrate immediately when the ECP was removed. The reason is that the bonding ability of the atoms inside the primary-carbide-like atomic clusters is strong because there are some covalent bonds between atoms in the M7C3 carbides, which are intermetallic compounds. The primary- carbides-like atomic clusters are not easily destroyed by temperature fluctuations and energy fluctuations in the melt because of the strong bonding. The excessive primary- carbides-like clusters caused by the ECP could survive for some time. It can be seen from equation (2) that the increase of carbide-like clusters can enhance the nucleation rate of primary carbides, and refine primary carbides. The excessive carbide-like clusters would gradually disintegrate under the effects of temperature fluctuations and energy fluctuations in the melt, if the temperature when the pulse current was removed were too high, which results in the insignificant effect of the ECP inoculation on the primary carbides. The higher the temperature, the worse the inoculation effect. On the contrary, the temperature of the ECP applied is closer to that of the primary phase precipitation, the less chance for the primary- carbides-like cluster to be disintegrated, the more beneficial for incubation. It is why the closer the temperature of the ECP removing is to the precipitation temperature of primary carbides, the number of primary carbide grains increases, and the grain size decreases, as shown in figure 5.
It is well known that austenite is a solid solution, the atoms are connected by metal bond, and the bonding force between atoms is relatively weak. Therefore, the bonding force between atoms in the austenite-like cluster is also relatively weak. The excessive austenite-like clusters are easily broken by temperature fluctuations and energy fluctuations in the melt when the ECP is removed. It should be the main reason why HOHCCI failed to show the incubation phenomenon, and It even resulted in that the primary austenite was not refined when the ECP was removed at a 5 °C superheat degree, see figure 3(e).
5. Conclusion
- 1.The ECP could not inoculate the primary austenite. The ECP could not inoculate primary M7C3 carbides when the ECP was removing at a 30 °C superheat degree, but it can inoculate primary M7C3 carbides when the ECP was applied until lower superheat.
- 2.Although the diffusion rate of the elements in the cluster is different, the rate is all relatively large compared with the time required for the general solidification process. It caused the solute to diffuse evenly within a short time after the ECP was removed. That is not an essential factor for the ECP incubation effect.
- 3.The main factor affecting the ECP incubation effect is the bonding strength between the internal atoms of the clusters associated with the primary phase. The stronger the bond strength of the corresponding atomic cluster, the more obvious the effect of inoculation.
Acknowledgments
Thanks for the financial support given by the Natural Science Foundation of China, the project No. of which is 51261011.









