Abstract
The magnetocaloric effect based Magnetic refrigeration (MR) was considered a novel energy-efficient and environmentally benign cooling method. However, the lack of suitable magnetic solids has slowed the development of its practical applications. We herein fabricated the RE2NiTiO6 (RE = Gd, Tb and Ho) double perovskite (DP) compounds and systematically determined their structural, magnetic and magnetocaloric properties by experimental determination and density functional theory calculations, in which the Gd2NiTiO6 was realized to exhibit promising cryogenic magnetocaloric performances. The results indicated that all the RE2NiTiO6 DP compounds crystallized in a distorted monoclinic structure with P21/n space group and underwent a second order type magnetic phase transition around 4.3, 4.5 and 3.9 K, for Gd2NiTiO6, Tb2NiTiO6 and Ho2NiTiO6, respectively. The magnetocaloric performances were checked by the parameters of maximum magnetic entropy change and relative cooling power, which are 31.28 J·kg−1·K−1 and 242.11 J·kg−1 for Gd2NiTiO6, 13.08 J·kg−1·K−1 and 213.41 J·kg−1 for Tb2NiTiO6, 11.98 J·kg−1·K−1 and 221.73 J·kg−1 for Ho2NiTiO6 under the magnetic field change of 0–50 kOe, respectively. Evidently, the Gd2NiTiO6 compound exhibit promising magnetocaloric performances and therefore is of potential for practical cryogenic MR applications.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 license. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
The development of novel magnetic materials with promising functional characters is of great significance for the advancement of modern technology and society. Among them, the magnetocaloric effect (MCE) based magnetic refrigeration (MR) technology promises to replace the conventional vapor cycle refrigeration, but also has a wide range for potential applications from room temperature to ultra-low temperature [1–6]. The MCE is a magnetic field induced thermodynamic phenomenon, which can be manifested as the heat generated or absorbed by the materials when the external magnetic field is changed. As a result, a large number of theoretical and experimental researches have been carried out on a variety of MR materials. Up to now, a series of MR materials with potential application near ambient temperature have been identified, such as Gd–Si–Ge [6], La–Fe–Si/Al [7], Mn–Fe–P–As/Ge/Si [8, 9], and Ni–Mn–X (X= Ga, In and Sn) [10, 11], etc. In comparison, rare earth (RE)-based compounds and oxides are regarded as the promising cryogenic MR materials for MR technology on account of their unique advantages and application prospects in the liquefaction of extremely low-temperature resources including helium (He), hydrogen (H2) and nitrogen (N2) [12–16].
In recent years, some of the RE-based oxides were fabricated and systematically checked, which are found to exhibit many intriguing physical properties in terms of magnetodielectric effect, magnetoresistive effect, luminescence characteristic and large/giant MCE effects, etc. For examples, Zhao et al have implemented the phase transition from antiferromagnetic-paraelectric to ferromagnetic–ferroelectric order in the (EuTiO3)0.5:(MgO)0.5 composite through the enhancement of spin-phonon coupling by negative pressure [17]. Arh et al have found that strong spin–orbit coupling (SOC) plays a key role in stabilizing spin liquid derived from magnetic anisotropy in the Ising triangular-lattice antiferromagnet NdTa7O19 oxide [18]. Blasco et al reported that each Fe2+ ion in the NaREFeWO6 (RE = Pr and Sm) oxides has strong antiferromagnetic coupling with the three nearest RE3+ ions and follows a unique magnetic order of 'up-up-down-down' sequence running along the [1 0 0] direction [19]. Moreover, Zeng et al have investigated the MCE performances in the weberite-type oxides Gd3
MO7 (M= Nb, Sb and Ta), and found that they are promising alternatives for MR techniques [20]. Fkhar et al have found the employment composite or spray drying as the effective strategy to improve magnetic properties in the La0.45Nd0.25Sr0.3MnO3/CuO compound [21, 22]. Koskelo et al have reported a series of fcc oxides A2GdSbO6 (A = Ca, Sr and Ba) with small superexchange interactions, the maximum magnetic entropy change ( ) are 20% higher than that of the standard MR material Gd3Ga5O12 using in extremely low-temperature [23]. Very recently, Xu et al have used the combination of experiment and density functional theory (DFT) calculation to reveal electronic, magnetic and MCE properties of the isostructural RE2BaZnO5 compounds, in which Dy2BaZnO5 and Ho2BaZnO5 compounds exhibit excellent magnetocaloric performances at liquid He temperature [24].
) are 20% higher than that of the standard MR material Gd3Ga5O12 using in extremely low-temperature [23]. Very recently, Xu et al have used the combination of experiment and density functional theory (DFT) calculation to reveal electronic, magnetic and MCE properties of the isostructural RE2BaZnO5 compounds, in which Dy2BaZnO5 and Ho2BaZnO5 compounds exhibit excellent magnetocaloric performances at liquid He temperature [24].
As an important branch of RE-based oxides, the RE2 TMTM'O6 (where TM and TM' are transition metal elements) double perovskite (DP) compounds derived from the ABO3 perovskite oxides have become the research focus in recent years. Because their intrinsic properties can be regulated by the variability of ions at RE, TM and TM' sites, they are endowed with outstanding optical, electrical, catalytic, magnetic and MCE properties [25–28]. In particular, the RE2 TMTM'O6 compounds have attracted extensive attention in exploring the exotic magnetic properties caused by strong SOC, structural variation diversity and anisotropic exchange interaction [29–31]. The competition of ferromagnetic and antiferromagnetic couplings among RE3+, Ni2+ and Ir4+ ions in the RE2NiIrO6 (RE = La, Pr and Nd) compounds can not only change the magnetic moment arrangement of Ni2+ and Ir4+ ions, but also increase the corresponding magnetically ordered temperatures with the decrease of the size of RE3+ [32]. The spontaneous magnetization of the RE2LiFeO6 (RE = Sm and Eu) compounds with abnormally high valence Fe5+ ions is closely related to the Dzyaloshinskii Moriya interaction, and the magnetization increases with increasing local geometric spin frustration between the nearest Fe5+ ions in the [1 1 1] direction [33]. In terms of MCE performances, the RE2 TMTM'O6 compounds commonly have lower hysteresis and higher chemical stability than rare earth (RE)-transition (TM) intermetallic compounds and alloys, as well as higher resistivity that facilitates reduced eddy current losses. Up to now, the excellent MCE performances in the field of extremely low-temperature have also been researched in the RE2CuMnO6 [34], RE2ZnMnO6 [35] and RE2FeAlO6 compounds [36]. It is evident from the above results that the exploration and investigation of the RE2 TMTM'O6 compounds with promising MCE performances is of great interest in the field of extremely low-temperatures MR, meanwhile, understanding its magnetic exchange interaction can also provide valuable theoretical information for the discipline of condensed matter physics.
Based on this background, we found that the RE2NiTiO6 compounds containing light RE ions have unique properties in the areas of optics, electricity and magnetism through the results of the relevant literatures [37–39]. However, there are few reports on the RE2NiTiO6 compounds containing heavy RE ions to date, especially in term of the magnetic and MCE properties. Therefore, we have studied the crystal structures, magnetic properties, electronic structures as well as MCE of the RE2NiTiO6 (RE = Gd, Tb and Ho) DP compounds by combining experiments with DFT calculations. As a consequence, we found that the anisotropic distortion in the monoclinic crystal structure of the RE2NiTiO6 compounds increase with decreasing RE3+ ion radius, resulting in the reduction of crystal symmetry. Furthermore, considerable reversible MCE performances have been observed in Gd2NiTiO6 compound, which provides a crucial clue for the exploration of MR materials suitable for extremely low-temperature.
2. Experimental and theoretical details
The RE2NiTiO6 (RE = Gd, Tb and Ho) polycrystalline compounds were prepared by citric acid-assisted sol-gel route. The precursor materials Gd(NO3)3 (⩾99.99%), Tb(NO3)3 (⩾99.99%), Dy(NO3)3 (⩾99.99%), Ni(NO3)2 (⩾99.99%) and Ti(SO4)2 (⩾99.99%) are purchased from the Shanghai Macklin Biochemical Co., Ltd. Firstly, stoichiometric amounts high purity raw materials of Gd(NO3)3, Tb(NO3)3 Dy(NO3)3, Ni(NO3)2 and Ti(SO4)2 were stirred in distilled water until they were completely dissolved. Subsequently, anhydrous citric acid was added to the precursor mixture solution. As a complex adhesive, anhydrous citric acid can help the good dispersion of various elements in the precursor gel. The obtained precursor mixture solutions were kept under constant magnetic stirring at 353 K for 12 h until viscous gels were formed. The gels were then fired at 1073 K for 2 h in an air atmosphere to remove organic residues and produce fluffy black powders. Finally, the black powders obtained after grinding were cold-pressed into pellets with the pressure of 30 MPa, and then annealed at 1473 K for 24 h. The phase characterization of the polycrystalline RE2NiTiO6 (RE = Gd, Tb and Ho) compounds were identified by the Rigaku x-ray diffraction technique (SmartLab-9 KW diffractometer). The surface morphology and chemical composition were investigated by using the field emission scanning electron microscope (FESEM, JEOL-JSM5800) and the attached energy dispersive X-ray spectroscopy (EDS). DC-susceptibility measurements of the RE2NiTiO6 (RE = Gd, Tb and Ho) compounds were conducted by the magnetic properties measurement system (MPMS, QD).
The structural optimizations, electronic and magnetic properties calculations were determined based on the projector augmented-wave (PAW) method within the DFT calculation by using the Vienna ab initio simulation package code [40, 41]. The spin-polarized generalized gradient approximation (GGA) was used to describe the exchange-correlation effects, and combined with both the GGA + U (Hubbard potential) and SOC to optimize the on-site Coulomb repulsion of localized RE-f electrons and determine the magnetic anisotropy, respectively [42, 43]. The value of Ueff with 3.4 eV has been set for Ni2+ ion according to the relation Ueff = U−J, where U and J are Coulomb and Hund's exchange parameters, respectively. The PAW method was used to resolve the electron–core interaction and the valence electron contributions for Gd, Tb, Ho, Ni, Ti and O were generated as [5s25p64f75d16s2], [5s25p64f95d0 6s2], [5s25p64f105d0 6s2], [3p63d84s2], [3p63d24s2] and [2s22p4], respectively [44, 45]. The kinetic energy cutoff was set at 600 eV to expansion the electronic wave functions. To optimize the structure, the conjugate gradient algorithm was used to completely relax the lattice constant and atomic position when the Hellman–Feynman forces converge to less than 0.01 eV·Å−1. The Brillouin zone was sampled using k-point mesh of 9 × 9 × 7 under the Monkhorst-Pack scheme, which can improve the accuracy of the density of states (DOS) and SOC calculations.
3. Results and discussion
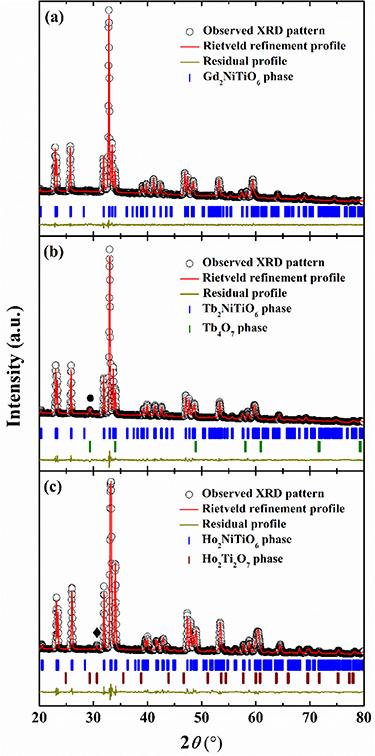
Figures 1(a)–(c) illustrate the obtained XRD patterns at room temperature and the Rietveld refinement results by aid of the FULLPROF software of the RE2NiTiO6 (RE = Gd, Tb and Ho) compounds. The present RE2NiTiO6 compounds are crystalized in the monoclinic structure with B-site ordered DP structure (P21/n space group, No. 14). In the Tb2NiTiO6 and Ho2NiTiO6 compounds, small amounts of Tb4O7 and Ho2Ti2O7 impurity phases were detected around 29° and 30.5° (the symbols ' 'and '
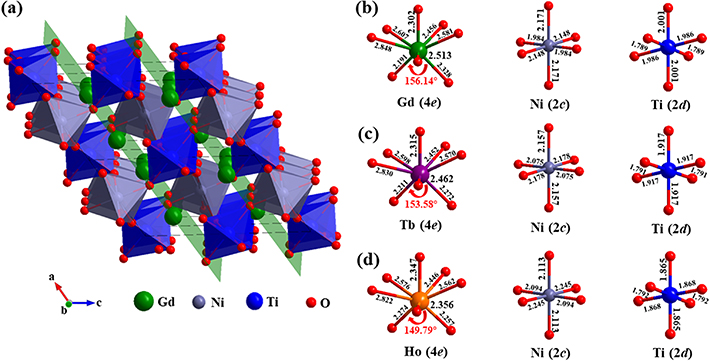
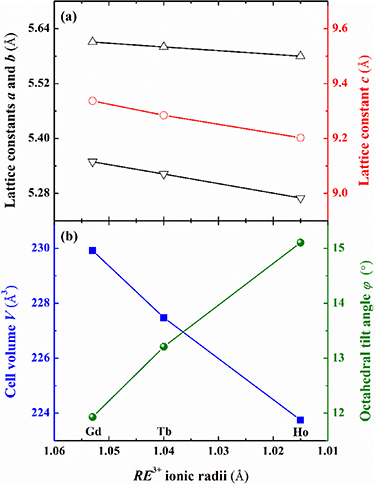
'and ' '), and the corresponding weight ratios were determined to be 1.83 and 3.25 wt%, respectively [46, 47]. The Rietveld indices were calculated, and the values of the refinement factors Rp, Rwp, Rexp and χ2 are all lower than 10% (as listed in table 1), which verifies the reliability of refinements. A schematic presentation of the crystal structure of the Gd2NiTiO6 compound along the b axis is illustrated in figures 2(a)–(d) with the environments of metal atom in RE2NiTiO6 compounds. The formation of the Gd2NiTiO6 compound superstructure makes the alternating distribution of the Ni located on the 2c and Ti on the 2d Wyckoff positions, which are surrounded by six O (4e) atoms to establish NiO6 and TiO6 octahedrons, respectively. Each TiO6 octahedron and the adjacent NiO6 octahedron share an O atom in the form of alternating z-shape chains along the a-axis. In parallel, Gd atoms are located on the 4e Wyckoff position, which not only occupy the cavities formed by co-point NiO6 and TiO6 octahedrons, but also superpose alternately with Ni/Ti atomic layers along the c-axis. In order to further distinguish the changes in crystal structure caused by different RE3+ ions, the refined lattice constants (a, b and c), the unit cell volume (V) and the average-octahedral-tilt angle (
'), and the corresponding weight ratios were determined to be 1.83 and 3.25 wt%, respectively [46, 47]. The Rietveld indices were calculated, and the values of the refinement factors Rp, Rwp, Rexp and χ2 are all lower than 10% (as listed in table 1), which verifies the reliability of refinements. A schematic presentation of the crystal structure of the Gd2NiTiO6 compound along the b axis is illustrated in figures 2(a)–(d) with the environments of metal atom in RE2NiTiO6 compounds. The formation of the Gd2NiTiO6 compound superstructure makes the alternating distribution of the Ni located on the 2c and Ti on the 2d Wyckoff positions, which are surrounded by six O (4e) atoms to establish NiO6 and TiO6 octahedrons, respectively. Each TiO6 octahedron and the adjacent NiO6 octahedron share an O atom in the form of alternating z-shape chains along the a-axis. In parallel, Gd atoms are located on the 4e Wyckoff position, which not only occupy the cavities formed by co-point NiO6 and TiO6 octahedrons, but also superpose alternately with Ni/Ti atomic layers along the c-axis. In order to further distinguish the changes in crystal structure caused by different RE3+ ions, the refined lattice constants (a, b and c), the unit cell volume (V) and the average-octahedral-tilt angle ( ) are illustrated in figures 3(a)–(c). Generally, the a, b, c and V decrease monotonically with decreasing RE3+ ions theoretical radii, obeying the principle of the lanthanide contraction. Moreover, the average-octahedral-tilt angle
) are illustrated in figures 3(a)–(c). Generally, the a, b, c and V decrease monotonically with decreasing RE3+ ions theoretical radii, obeying the principle of the lanthanide contraction. Moreover, the average-octahedral-tilt angle  can reflect the degree of anisotropic distortion of the unit cell in monoclinic compounds. The
can reflect the degree of anisotropic distortion of the unit cell in monoclinic compounds. The  is defined as the evolution of the average tilting angle ∠Ni–O–Ti and the corresponding values are 156.14°, 153.58° and 149.79° for RE = Gd, Tb and Ho. The values of
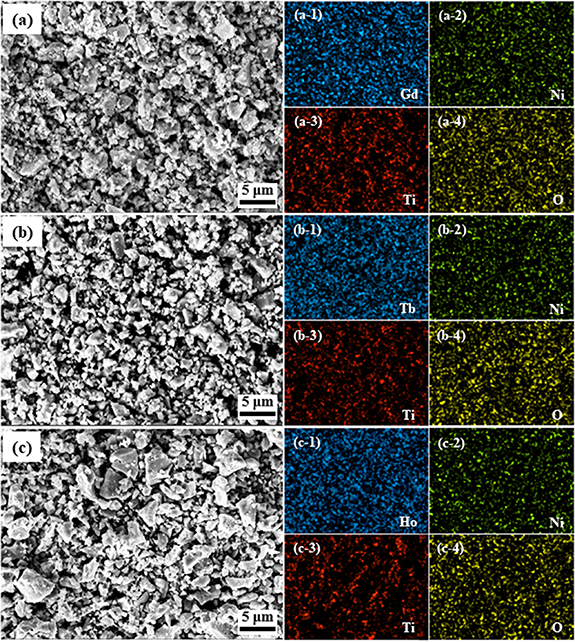
is defined as the evolution of the average tilting angle ∠Ni–O–Ti and the corresponding values are 156.14°, 153.58° and 149.79° for RE = Gd, Tb and Ho. The values of  suggest that the monoclinic distortion of the present RE2NiTiO6 compounds will increase monotonically with the decrease of the radii of RE3+ ions and result in the reduction of crystal symmetry. The FESEM micrographs for the present RE2NiTiO6 compounds together with the corresponding EDS mappings of each element are presented in figures 4(a)–(c). The RE2NiTiO6 (RE = Gd, Tb and Ho) compounds are composed of uniformly distributed irregular particles with average particle sizes about 1.82, 1.86 and 1.87 μm. This microstructure with irregular particles and high density confirms the polycrystalline nature of the RE2NiTiO6 compounds. The randomly selected regions of EDS results analysis verified that the atomic ratios of the present RE2NiTiO6 series compounds are close to the standard 2:1:1:6. The ratios of each element are 20.16, 10.99, 10.68, 58.17 at %, 21.13, 9.22, 10.91, 58.74 at % and 21.19, 11.05, 11.09, 56.67 at % for Gd2NiTiO6, Tb2NiTiO6 and Ho2NiTiO6 compounds, respectively.
suggest that the monoclinic distortion of the present RE2NiTiO6 compounds will increase monotonically with the decrease of the radii of RE3+ ions and result in the reduction of crystal symmetry. The FESEM micrographs for the present RE2NiTiO6 compounds together with the corresponding EDS mappings of each element are presented in figures 4(a)–(c). The RE2NiTiO6 (RE = Gd, Tb and Ho) compounds are composed of uniformly distributed irregular particles with average particle sizes about 1.82, 1.86 and 1.87 μm. This microstructure with irregular particles and high density confirms the polycrystalline nature of the RE2NiTiO6 compounds. The randomly selected regions of EDS results analysis verified that the atomic ratios of the present RE2NiTiO6 series compounds are close to the standard 2:1:1:6. The ratios of each element are 20.16, 10.99, 10.68, 58.17 at %, 21.13, 9.22, 10.91, 58.74 at % and 21.19, 11.05, 11.09, 56.67 at % for Gd2NiTiO6, Tb2NiTiO6 and Ho2NiTiO6 compounds, respectively.
Figure 1. The XRD patterns for the (a) Gd2NiTiO6, (b) Tb2NiTiO6 and (c) Ho2NiTiO6 compounds.
Download figure:
Standard image High-resolution imageFigure 2. The crystal structure along the b axis (a) of the Gd2NiTiO6 compound and the environments of metal atomic (b)–(d) for the RE2NiTiO6 (RE = Gd, Tb and Ho) compounds.
Download figure:
Standard image High-resolution imageFigure 3. The lattice constants and octahedral tilt angles of the RE2NiTiO6 compounds.
Download figure:
Standard image High-resolution imageFigure 4. The SEM images and the distributions of RE, Ni, Ti and O elements for the (a) Gd2NiTiO6, (b) Tb2NiTiO6 and (c) Ho2NiTiO6 compounds.
Download figure:
Standard image High-resolution imageTable 1. The lattice constants, refinement factors and the positional parameters for the present RE2NiTiO6 compounds.
| Parameters | Gd2NiTiO6 | Tb2NiTiO6 | Ho2NiTiO6 | |
|---|---|---|---|---|
| a (Å) | 5.34(3) | 5.32(2) | 5.27(5) | |
| b (Å) | 5.61(5) | 5.60(1) | 5.59(1) | |
| c (Å) | 9.33(3) | 9.28(4) | 9.20(2) | |
| β (°) | 124.86(2) | 124.77(5) | 124.52(4) | |
| V (Å3) | 229.91 | 227.47 | 223.75 | |
| Reliability factors | ||||
| Rp (%) | 1.99 | 3.02 | 3.14 | |
| Rwp (%) | 2.50 | 3.92 | 4.07 | |
| Rexp (%) | 1.87 | 1.77 | 1.12 | |
| χ2 | 1.79 | 4.89 | 9.88 | |
| Atomic position | ||||
| RE | 4e (x y z) | |||
| x | 0.2312(6) | 0.2311(2) | 0.2276(2) | |
| y | 0.5636(3) | 0.5634(5) | 0.5669(6) | |
| z | 0.7469(5) | 0.7469(1) | 0.7444(4) | |
| Ni | 2c (0 1/2 0) | |||
| Ti | 2d (1/2 0 0) | |||
| O1 | 4e (x y z) | |||
| x | 0.1491(1) | 0.1398(5) | 0.1361(4) | |
| y | 0.7356(4) | 0.7333(3) | 0.7152(6) | |
| z | 0.4678(4) | 0.4622(1) | 0.4600(5) | |
| O2 | 4e (x y z) | |||
| x | 0.2240(1) | 0.2196(4) | 0.2343(2) | |
| y | 0.1849(5) | 0.1807(6) | 0.1754(3) | |
| z | 0.4256(6) | 0.4302(3) | 0.4367(5) | |
| O3 | 4e (x y z) | |||
| x | 0.3451(2) | 0.3452(1) | 0.3358(4) | |
| y | 0.5396(2) | 0.5393(6) | 0.5433(4) | |
| z | 0.2510(5) | 0.2389(2) | 0.2337(2) |
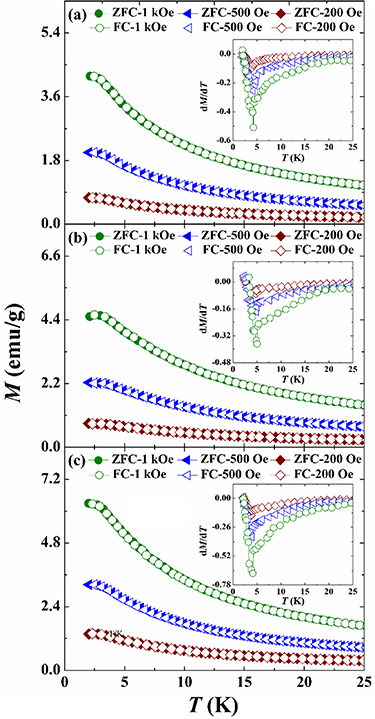
The temperature dependent magnetization, the M(T) curves for the RE2NiTiO6 compounds are illustrated in figures 5(a)–(c), which were carried out in zero-field-cooled (ZFC)/field-cooled (FC) modes under the magnetic flux densities ( ) of 200, 500 and 1000 Oe. All the M(T) curves of present RE2NiTiO6 compounds increase with the increase
) of 200, 500 and 1000 Oe. All the M(T) curves of present RE2NiTiO6 compounds increase with the increase  in the whole temperature range. Another feature observed in each M(T) curve under different
in the whole temperature range. Another feature observed in each M(T) curve under different  is that ZFC and FC modes have good consistency, which confirms these compounds have negligible thermal hysteresis. From a technical point of view, the perfect reversibility of magnetic phase transitions (MPTs) makes the present RE2NiTiO6 series compounds have potential application prospects of extremely low-temperature MR technology. Insets are the derivative curves of the corresponding M(T) curves for the RE2NiTiO6 compounds. It can be observed that the minimum values of all the RE2NiTiO6 compounds shift toward higher temperatures with increasing
is that ZFC and FC modes have good consistency, which confirms these compounds have negligible thermal hysteresis. From a technical point of view, the perfect reversibility of magnetic phase transitions (MPTs) makes the present RE2NiTiO6 series compounds have potential application prospects of extremely low-temperature MR technology. Insets are the derivative curves of the corresponding M(T) curves for the RE2NiTiO6 compounds. It can be observed that the minimum values of all the RE2NiTiO6 compounds shift toward higher temperatures with increasing  , implying that their dominant MPTs are of the FM type. When
, implying that their dominant MPTs are of the FM type. When  increases to 1000 Oe, the magnetic ordering temperatures (TM) are determined to be 4.3, 4.5 and 3.9 K, which are obtained from the minimum values of the temperature axes for the Gd2NiTiO6, Tb2NiTiO6 and Ho2NiTiO6 compounds, respectively.
increases to 1000 Oe, the magnetic ordering temperatures (TM) are determined to be 4.3, 4.5 and 3.9 K, which are obtained from the minimum values of the temperature axes for the Gd2NiTiO6, Tb2NiTiO6 and Ho2NiTiO6 compounds, respectively.
Figure 5. The M(T) curves of the (a) Gd2NiTiO6, (b) Tb2NiTiO6 and Ho2NiTiO6 compounds in the presence of  = 200, 500 and 1000 Oe. Insets are the derivative curves of the corresponding M(T) curves.
= 200, 500 and 1000 Oe. Insets are the derivative curves of the corresponding M(T) curves.
Download figure:
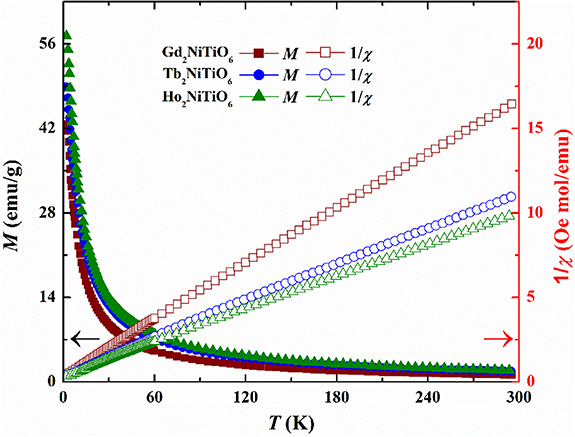
Standard image High-resolution imageThe magnetization and inverse magnetic susceptibility ( ) of present RE2NiTiO6 compounds are plotted as a function of temperature in the presence of magnetic flux densities
) of present RE2NiTiO6 compounds are plotted as a function of temperature in the presence of magnetic flux densities  = 10 kOe, as illustrated in figures 6(a)–(c). In the high-temperature part (50 ⩽ T ⩽ 300 K), all the χ−1(T) curves of present RE2NiTiO6 compounds are almost linear, which satisfies the modified Curie-Weiss law:
= 10 kOe, as illustrated in figures 6(a)–(c). In the high-temperature part (50 ⩽ T ⩽ 300 K), all the χ−1(T) curves of present RE2NiTiO6 compounds are almost linear, which satisfies the modified Curie-Weiss law:  , in which
, in which  denotes the Curie constant,
denotes the Curie constant,  and
and  are named as paramagnetic Curie temperature and effective magnetic moment, respectively. The values of
are named as paramagnetic Curie temperature and effective magnetic moment, respectively. The values of  are calculated as 11.11
are calculated as 11.11  for Gd2NiTiO6, 13.42
for Gd2NiTiO6, 13.42  for Tb2NiTiO6, as well as 14.89
for Tb2NiTiO6, as well as 14.89  for Ho2NiTiO6, which are significantly larger than the values of theoretical magnetic moments provided by free Gd3+ (7.94
for Ho2NiTiO6, which are significantly larger than the values of theoretical magnetic moments provided by free Gd3+ (7.94  ), Tb3+ (9.72
), Tb3+ (9.72  ) and Ho3+ (10.64
) and Ho3+ (10.64  ) ions, respectively. This result implies the magnetic moment contribution of Ni2+ should be considered. In this regard, the values of
) ions, respectively. This result implies the magnetic moment contribution of Ni2+ should be considered. In this regard, the values of  are compared with the theoretical values calculated by the formula
are compared with the theoretical values calculated by the formula ![${\mu _{{\text{theor}}}} = {\left[ {2\mu {{(R{E^{3 + }})}^2} + \mu {{({\text{N}}{{\text{i}}^{2 + }})}^2}} \right]^{{1 \mathord{\left/ \right. } 2}}}$](https://content.cld.iop.org/journals/2515-7655/5/1/014017/revision3/jpenergyacb176ieqn27.gif) , and the gained quite close values establish the existence of the spin-only magnetic moments of Ni2+ (2
, and the gained quite close values establish the existence of the spin-only magnetic moments of Ni2+ (2  ).
).
Figure 6. The M(T) and  curves of the present RE2NiTiO6 compounds in the presence of
curves of the present RE2NiTiO6 compounds in the presence of  = 10 kOe.
= 10 kOe.
Download figure:
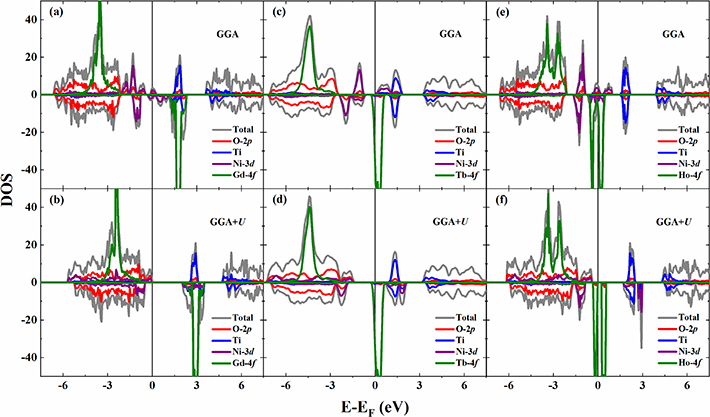
Standard image High-resolution imageTo further understand the magnetic properties and electronic structures of the RE2NiTiO6 compounds in the ground states, the total and partial DOS have been evaluated by the GGA and GGA + U methods in the framework of ab initio calculation, as illustrated in figures 7(a)–(f). It can be seen that the Gd2NiTiO6 and Ho2NiTiO6 compounds behave as semiconductors in the GGA+U method, with the band gaps of 1.83 and 0.25 eV, respectively. The Tb2NiTiO6 compound is semi-metallic in both GGA and GGA+U methods. Moreover, the magnetic properties of all compounds are mainly provided by RE3+ and Ni2+ ions. The Gd2NiTiO6 and Ho2NiTiO6 compounds have FM ground states, while the Tb2NiTiO6 compound has an AFM ground state contributing from the antiparallel arrangement of the Ni2+ ion. The atomic magnetic moments ( ) of the present RE2NiTiO6 series compounds have been determined by spin-polarized calculation according to the obtained magnetic ground states. The
) of the present RE2NiTiO6 series compounds have been determined by spin-polarized calculation according to the obtained magnetic ground states. The  values of each RE3+ and Ni2+ ion are determined to be 6.83 and 1.65
values of each RE3+ and Ni2+ ion are determined to be 6.83 and 1.65  /atom for the Gd2NiTiO6 compound, 5.81 and 1.66
/atom for the Gd2NiTiO6 compound, 5.81 and 1.66  /atom for the Tb2NiTiO6 compound, as well as 3.75 and 1.65
/atom for the Tb2NiTiO6 compound, as well as 3.75 and 1.65  /atom for the Ho2NiTiO6 compound, respectively. Moreover, the magnetic anisotropy energies (
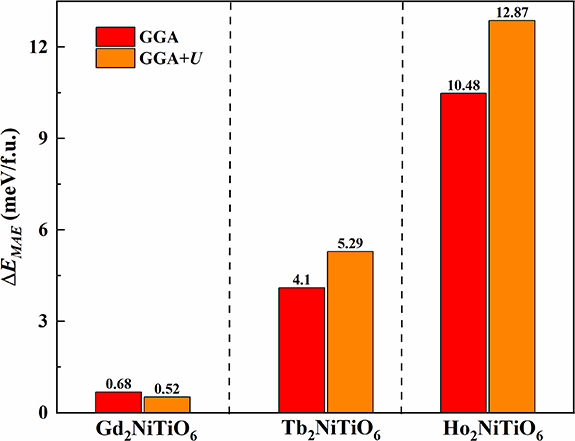
/atom for the Ho2NiTiO6 compound, respectively. Moreover, the magnetic anisotropy energies ( ) of the present RE2NiTiO6 series compounds have been further investigated based on the SOC method. The
) of the present RE2NiTiO6 series compounds have been further investigated based on the SOC method. The  is defined as the difference of energy in various directions, which can be formulated by the equation:
is defined as the difference of energy in various directions, which can be formulated by the equation:  . Where
. Where  and
and  are expressed as the maximum and minimum energies of the quantum axes along the [0 0 1], [0 1 0] and [1 0 0] directions, respectively. The acquired results of the present RE2NiTiO6 series compounds are presented as bar charts in figure 8, where the red and orange blocks are corresponding to the GGA and GGA+U methods, respectively. Obviously, the values of
are expressed as the maximum and minimum energies of the quantum axes along the [0 0 1], [0 1 0] and [1 0 0] directions, respectively. The acquired results of the present RE2NiTiO6 series compounds are presented as bar charts in figure 8, where the red and orange blocks are corresponding to the GGA and GGA+U methods, respectively. Obviously, the values of  calculated by both methods exhibit gradual increase, which indicates that the magnetic moments of the present RE2NiTiO6 series compounds decrease with the increase of
calculated by both methods exhibit gradual increase, which indicates that the magnetic moments of the present RE2NiTiO6 series compounds decrease with the increase of  . In addition, the values of
. In addition, the values of  for the Gd2NiTiO6 compound in both methods are close to zero, demonstrating that the
for the Gd2NiTiO6 compound in both methods are close to zero, demonstrating that the  of Ni2+ ions in all RE2NiTiO6 compounds are extremely small. Hence, it can conclude that the
of Ni2+ ions in all RE2NiTiO6 compounds are extremely small. Hence, it can conclude that the  of the Tb2NiTiO6 and Ho2NiTiO6 compounds are mainly caused by Tb3+ and Ho3+ ions.
of the Tb2NiTiO6 and Ho2NiTiO6 compounds are mainly caused by Tb3+ and Ho3+ ions.
Figure 7. The calculated density of states (DOS) at ground states for (a) and (b) Gd2NiTiO6, (c) and (d) Tb2NiTiO6 as well as (e) and (f) Ho2NiTiO6 compounds.
Download figure:
Standard image High-resolution imageFigure 8. The magnetic anisotropy energy of the RE2NiTiO6 compounds obtained by GGA and GGA+U methods.
Download figure:
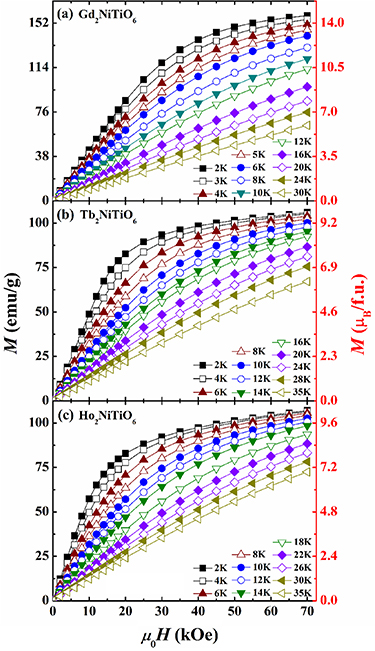
Standard image High-resolution imageA series of  curves for present RE2NiTiO6 compounds are recorded at 2–35 K with
curves for present RE2NiTiO6 compounds are recorded at 2–35 K with  up to 70 kOe, as presented in figures 9(a)–(c). Generally, the
up to 70 kOe, as presented in figures 9(a)–(c). Generally, the  curves of all compounds at 2 K tend to be saturated with the increasing
curves of all compounds at 2 K tend to be saturated with the increasing  . The saturation magnetization (
. The saturation magnetization ( ) values are determined to be 14.67, 9.87 and 10.21
) values are determined to be 14.67, 9.87 and 10.21  for the Gd2NiTiO6, Tb2NiTiO6 and Ho2NiTiO6 compounds, respectively. In the case of Gd2NiTiO6 compound, the value of
for the Gd2NiTiO6, Tb2NiTiO6 and Ho2NiTiO6 compounds, respectively. In the case of Gd2NiTiO6 compound, the value of  approximates to the sum of two isolated Gd3+ ions and one Ni2+ ion. In contrast, the values of
approximates to the sum of two isolated Gd3+ ions and one Ni2+ ion. In contrast, the values of  deviate from the individual magnetic ions for the Tb2NiTiO6 and Ho2NiTiO6 compounds, which may be due to the non-negligible magnetic anisotropy [48, 49]. The temperature dependent magnetic entropy change (
deviate from the individual magnetic ions for the Tb2NiTiO6 and Ho2NiTiO6 compounds, which may be due to the non-negligible magnetic anisotropy [48, 49]. The temperature dependent magnetic entropy change ( ) curve provides crucial information about the present RE2NiTiO6 series compounds, which can not only verify whether they are suitable for the application of MR technology, but also further judge their MPTs type. The
) curve provides crucial information about the present RE2NiTiO6 series compounds, which can not only verify whether they are suitable for the application of MR technology, but also further judge their MPTs type. The  is given by the following formula based on thermodynamic theory [50]:
is given by the following formula based on thermodynamic theory [50]:

Figure 9. The  curves of the (a) Gd2NiTiO6, (b) Tb2NiTiO6 and Ho2NiTiO6 compounds.
curves of the (a) Gd2NiTiO6, (b) Tb2NiTiO6 and Ho2NiTiO6 compounds.
Download figure:
Standard image High-resolution imageFrom the well measured  curve, the
curve, the  can be approximately evaluated by the following expression:
can be approximately evaluated by the following expression:
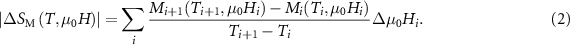
Here, Mi
and Mi+
1 can be obtained by the magnetization at the corresponding Ti
and Ti+
1 when a certain  is fixed. According to equation (2), the resulting
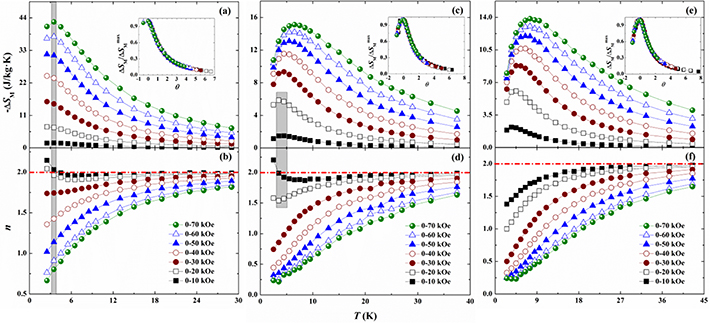
is fixed. According to equation (2), the resulting  curves for the RE2NiTiO6 compounds collected at several typical are presented in figures 10 (a), (c) and (e). The maximal values (
curves for the RE2NiTiO6 compounds collected at several typical are presented in figures 10 (a), (c) and (e). The maximal values ( ) of the present RE2NiTiO6 series compounds around TM increase with the increase of
) of the present RE2NiTiO6 series compounds around TM increase with the increase of  and gradually shift to high temperatures, which is consistent with the MR materials with typical second-ordering MPT characteristics. The values of
and gradually shift to high temperatures, which is consistent with the MR materials with typical second-ordering MPT characteristics. The values of  around TM with
around TM with  of 0–20 and 0–50 kOe were calculated to be 6.92 and 31.28 J·kg−1·K−1 for the Gd2NiTiO6 compound, 5.86 and 13.08 J·kg−1·K−1 for the Tb2NiTiO6 compound as well as 6.08 and 11.98 J·kg−1·K−1 for the Ho2NiTiO6 compound, respectively. In view of the strong correlation between the MCE and MPT of magnetic solid in the actual MR cycle, we can analyze the
of 0–20 and 0–50 kOe were calculated to be 6.92 and 31.28 J·kg−1·K−1 for the Gd2NiTiO6 compound, 5.86 and 13.08 J·kg−1·K−1 for the Tb2NiTiO6 compound as well as 6.08 and 11.98 J·kg−1·K−1 for the Ho2NiTiO6 compound, respectively. In view of the strong correlation between the MCE and MPT of magnetic solid in the actual MR cycle, we can analyze the  curves to determine the types of first/second-ordering (FO/SO) MPT. The scaling relations have become a universal model for determining MR material with the nature of SO-MPT, which is expressed as assembling the
curves to determine the types of first/second-ordering (FO/SO) MPT. The scaling relations have become a universal model for determining MR material with the nature of SO-MPT, which is expressed as assembling the  curves into a single master curve [51, 52]. The insets of figures 10 (a), (c) and (e) illustrate the rescaled temperature
curves into a single master curve [51, 52]. The insets of figures 10 (a), (c) and (e) illustrate the rescaled temperature  (
( ) dependent
) dependent  (
( ) curves by conducting the normalizations around TM for the present RE2NiTiO6 series compounds, where
) curves by conducting the normalizations around TM for the present RE2NiTiO6 series compounds, where  (above TM) and
(above TM) and  (below TM) are two reference points corresponding to
(below TM) are two reference points corresponding to  . It can be found from the normalization
. It can be found from the normalization  curves that the right part can converge to one single curve when the present RE2NiTiO6 compounds take TM as the symmetry point, which indicates that they have the characteristics of SO-MPTs around TM. Since the temperatures of the present RE2NiTiO6 compounds below TM are close to the absolute zero, the left part of the normalization
curves that the right part can converge to one single curve when the present RE2NiTiO6 compounds take TM as the symmetry point, which indicates that they have the characteristics of SO-MPTs around TM. Since the temperatures of the present RE2NiTiO6 compounds below TM are close to the absolute zero, the left part of the normalization  curves do not in accordance with the scaling relations, so that it is difficult to confirm the type of MPT. Accordingly, a novel criterion for the relationship between MCE and the exponent n proposed by Law et al [53, 54] was used for verification research, which can be expressed as following formula:
curves do not in accordance with the scaling relations, so that it is difficult to confirm the type of MPT. Accordingly, a novel criterion for the relationship between MCE and the exponent n proposed by Law et al [53, 54] was used for verification research, which can be expressed as following formula:

Figure 10. The  curves and the corresponding n(T) curves of the present RE2NiTiO6 compounds. Insets of (a), (c) and (e) show the modified plots of
curves and the corresponding n(T) curves of the present RE2NiTiO6 compounds. Insets of (a), (c) and (e) show the modified plots of  versus
versus  .
.
Download figure:
Standard image High-resolution imageThe n(T) curves converted from the  curves for the present RE2NiTiO6 series compounds collected at
curves for the present RE2NiTiO6 series compounds collected at  up to 0–70 kOe are illustrated in figure 10(b), (d) and (f). Scrutiny over the MPTs type of present RE2NiTiO6 compounds reveals that the exponents of n are less than 2 around TM with the further increase of
up to 0–70 kOe are illustrated in figure 10(b), (d) and (f). Scrutiny over the MPTs type of present RE2NiTiO6 compounds reveals that the exponents of n are less than 2 around TM with the further increase of  , indicating the nature of SO-MPT. In addition, two or one points in n value are larger than 2 below TM can be found in Gd2NiTiO6 and Tb2NiTiO6 compounds, which is probability owing to the instability of their magnetic ground states close to absolute zero.
, indicating the nature of SO-MPT. In addition, two or one points in n value are larger than 2 below TM can be found in Gd2NiTiO6 and Tb2NiTiO6 compounds, which is probability owing to the instability of their magnetic ground states close to absolute zero.
In addition, the parameters of temperature averaged  , relative cooling power (RCP) and refrigerant capacity (RC) obtained from the
, relative cooling power (RCP) and refrigerant capacity (RC) obtained from the  curve can also be used as variety effective ways to evaluate the MCE performance, which can be calculated by the following formulas:
curve can also be used as variety effective ways to evaluate the MCE performance, which can be calculated by the following formulas:
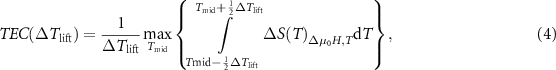


Griffith et al defined the temperature averaged entropy change (TEC) as the average value of  curve in a selected temperature range (
curve in a selected temperature range ( ) under a fixed
) under a fixed  , and
, and  is expressed as the center of the
is expressed as the center of the  [55]. According to equation (4), the resulting values of TEC (5 K) were calculated to be 5.88 and 27.38 J·kg−1·K−1 for the Gd2NiTiO6 compound, 5.24 and 12.71 J·kg−1·K−1 for the Tb2NiTiO6 compound, as well as 5.62 and 11.72 J·kg−1·K−1 for the Ho2NiTiO6 compound with the
[55]. According to equation (4), the resulting values of TEC (5 K) were calculated to be 5.88 and 27.38 J·kg−1·K−1 for the Gd2NiTiO6 compound, 5.24 and 12.71 J·kg−1·K−1 for the Tb2NiTiO6 compound, as well as 5.62 and 11.72 J·kg−1·K−1 for the Ho2NiTiO6 compound with the  of 0–20 and 0–50 kOe, respectively. It can be observed that the values of TEC (5 K) deviate greatly from the corresponding
of 0–20 and 0–50 kOe, respectively. It can be observed that the values of TEC (5 K) deviate greatly from the corresponding  with the same
with the same  , which is particularly prominent in the Gd2NiTiO6 compound. Such behavior is related to the narrow temperature span of the
, which is particularly prominent in the Gd2NiTiO6 compound. Such behavior is related to the narrow temperature span of the  curve for Gd2NiTiO6 compound. The RCP and RC are commonly used as the coefficients of MCE performance, which can reliably predict the amounts of energy transferred from Tcold to Thot in the MR cycle. The Tcold and Thot are expressed as the two sides at
curve for Gd2NiTiO6 compound. The RCP and RC are commonly used as the coefficients of MCE performance, which can reliably predict the amounts of energy transferred from Tcold to Thot in the MR cycle. The Tcold and Thot are expressed as the two sides at  values of
values of  curves collected at several types of
curves collected at several types of  , and the value of
, and the value of  is equal to Thot
–Tcold. According to equations (5) and (6), the calculated values of RCP (RC) collected at
is equal to Thot
–Tcold. According to equations (5) and (6), the calculated values of RCP (RC) collected at  up to 0–20 and 0–50 kOe were 45.44 (34.79) and 242.11 (185.24) J·kg−1 for the Gd2NiTiO6 compound, 49.15 (38.21) and 213.41 (168.36) J·kg−1 for the Tb2NiTiO6 compound, as well as 57.33 (45.27) and 221.73 (174.56) J·kg−1 for the Ho2NiTiO6 compound, respectively. Moreover, the calculated values of
up to 0–20 and 0–50 kOe were 45.44 (34.79) and 242.11 (185.24) J·kg−1 for the Gd2NiTiO6 compound, 49.15 (38.21) and 213.41 (168.36) J·kg−1 for the Tb2NiTiO6 compound, as well as 57.33 (45.27) and 221.73 (174.56) J·kg−1 for the Ho2NiTiO6 compound, respectively. Moreover, the calculated values of  , RC and RCP under the
, RC and RCP under the  of 0–50 kOe for the present RE2NiTiO6 series compounds together with some reported promising RE-based MR materials with TM below 10 K are summarized in table 2. It is evident that the present RE2NiTiO6 series compounds can be used as excellent MR materials in the extremely low-temperature region. In particular, the maximal value (
of 0–50 kOe for the present RE2NiTiO6 series compounds together with some reported promising RE-based MR materials with TM below 10 K are summarized in table 2. It is evident that the present RE2NiTiO6 series compounds can be used as excellent MR materials in the extremely low-temperature region. In particular, the maximal value ( = 42.64 J·kg−1·K−1,
= 42.64 J·kg−1·K−1,  = 0–70 kOe) of the Gd2NiTiO6 compound is quite impressive when compared to the benchmark of commercial application, Gd3Ga5O12 (
= 0–70 kOe) of the Gd2NiTiO6 compound is quite impressive when compared to the benchmark of commercial application, Gd3Ga5O12 ( = 38.4 J·kg−1·K−1,
= 38.4 J·kg−1·K−1,  = 0–70 kOe).
= 0–70 kOe).
Table 2. The values of  , TEC (5 K), RCP and RC with the
, TEC (5 K), RCP and RC with the  of 0–50 kOe for present RE2NiTiO6 compounds and some reported promising RE-based MR materials.
of 0–50 kOe for present RE2NiTiO6 compounds and some reported promising RE-based MR materials.
| Material | TM (K) |
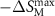 (J·kg−1·K−1) (J·kg−1·K−1) | TEC (5 K) (J·kg−1·K−1) | RCP (J·kg−1) | RC (J·kg−1) | References |
|---|---|---|---|---|---|---|
| Gd2NiTiO6 | 4.3 | 31.28 | 27.38 | 242.11 | 185.24 | present |
| Tb2NiTiO6 | 4.5 | 13.08 | 12.71 | 213.41 | 168.36 | present |
| Ho2NiTiO6 | 3.9 | 11.98 | 11.72 | 221.73 | 174.56 | present |
| Gd2ZnMnO6 | 6.4 | 15.1 | — | 226.2 | 176.8 | [35] |
| Gd2FeAlO6 | 3.5 | 19.2 | — | — | 136.1 | [36] |
| Sr2GdNbO6 | 2.0 | 26.07 | 19.02 | 195.5 | 148.2 | [5] |
| GdFe2Si2 | 8.6 | 23.25 | 21.01 | 276.56 | 205.67 | [56] |
| TbScO3 | 3.1 | 23.71 | — | — | — | [57] |
| GdCrTiO5 | 5 | 25.1 | — | — | — | [58] |
| Er2FeC4 | 6 | 21.62 | — | — | 280.3 | [59] |
| ErRu2Si2 | 5.5 | 17.6 | — | — | — | [60] |
| HoCuSi | 7 | 33.1 | — | — | 385 | [61] |
4. Conclusions
To conclude, we have successfully fabricated the polycrystalline RE2NiTiO6 (RE = Gd, Tb and Ho) DP compounds and their crystallographic structures, electronic structures, magnetic properties and MCE were investigated in combination with experiment and theory. All the RE2NiTiO6 compounds are confirmed to crystallize in monoclinic structure with P21/n space group by XRD refinements. The ground states of all compounds are FM ordering around TM, and the characteristics of SO-MPTs are further confirmed by the novel criterion of n(T) curves and the normalization  curves. The MCEs are determined by an indirect approach of experimental data, which yields the
curves. The MCEs are determined by an indirect approach of experimental data, which yields the  , TEC (5 K), RCP and RC values with
, TEC (5 K), RCP and RC values with  of 0–70 kOe as high as 42.64 J·kg−1·K−1, 38.84 J·kg−1·K−1, 419.36 J·kg−1 and 321.66 J·kg−1 for the Gd2NiTiO6 compound, 15.12 J·kg−1·K−1, 14.85 J·kg−1·K−1, 330.38 J·kg−1 and 259.81 J·kg−1 for the Tb2NiTiO6 compound, as well as 13.85 J·kg−1·K−1, 13.63 J·kg−1·K−1, 346.18 J·kg−1 and 269.15 J·kg−1 for the Ho2NiTiO6 compound, respectively. Compared with the reported RE-based MR materials, the superior MCE performances of the Gd2NiTiO6 compound make it an excellent candidate as MR material suitable for extremely low-temperature.
of 0–70 kOe as high as 42.64 J·kg−1·K−1, 38.84 J·kg−1·K−1, 419.36 J·kg−1 and 321.66 J·kg−1 for the Gd2NiTiO6 compound, 15.12 J·kg−1·K−1, 14.85 J·kg−1·K−1, 330.38 J·kg−1 and 259.81 J·kg−1 for the Tb2NiTiO6 compound, as well as 13.85 J·kg−1·K−1, 13.63 J·kg−1·K−1, 346.18 J·kg−1 and 269.15 J·kg−1 for the Ho2NiTiO6 compound, respectively. Compared with the reported RE-based MR materials, the superior MCE performances of the Gd2NiTiO6 compound make it an excellent candidate as MR material suitable for extremely low-temperature.
Acknowledgment
The present work was supported by the National Natural Science Foundation of China (Grant No. 52171174). The authors acknowledge the Supercomputing Center of Hangzhou Dianzi University for providing computing resources.
Data availability statement
All data that support the findings of this study are included within the article (and any supplementary files).
Conflict of interest
There are no conflicts to declare.