Abstract
We have investigated plasmon-assisted energy conversion in dye-sensitized solar cells (DSCs) applying gold nanoparticles (NPs) modified fluorine tin oxide (FTO) electrodes. A series of Au NPs with different sizes (15–80 nm) were synthesized and immobilized onto FTO glass slides. Photoanodes were prepared on these Au modified FTO substrates using P25 TiO2 powders and by the screen-printing method. The size effects of Au NPs on the photovoltaic performance of the formed DSCs were investigated systematically. Structural and photoelectrochemical properties of the formed photoanodes were examined by field emission scanning electron microscopy and electrochemical impedance spectroscopy. It was found that the energy conversion efficiency of the DSC was highly dependent on the Au particle size. When the particle size was not greater than 60 nm, the DSC based on the Au NP-FTO composite electrode showed a higher short-circuit current density and better photovoltaic (PV) performance than the cell based on the bare FTO. The best cell was achieved using 25 nm sized Au NPs modified FTO. It exhibited a conversion efficiency of 6.69%, which was 15% higher than that of DSCs without Au NPs. The related PV performance enhancement mechanisms, photoelectrochemical processes and surface-plasmon resonances in DSCs with Au nanostructures are analysed and discussed.
Export citation and abstract BibTeX RIS
1. Introduction
Dye-sensitized solar cells (DSCs), which are one of the most potential photovoltaic (PV) devices, have attracted tremendous interest as an alternative technology for future renewable energy production due to their high solar energy conversion efficiency as well as relatively low fabrication cost [1, 2]. Currently, diverse research effort to improve the overall conversion efficiency has been directed at engineering the anode, cathode, dye and redox shuttle. For example, coating mesoporous TiO2 networks with various types of insulating metal oxides [3, 4], replacing TiO2 by other semiconducting oxides [5], synthesizing new dyes that broadly and effectively absorb visible and near-infrared light [6, 7], optimization of semiconducting titanium dioxide (TiO2) nanostructures, which strongly depend on their dimensional (e.g. size and shape) and morphological features, for facial dye loading and electron injection into the conduction band and electrode with a quantum yield of unit [8, 9], synthesizing alternative redox couples [10, 11], the replacement of Pt counter electrodes (CEs) with less expensive and electrochemically stable elements [12] and guarantee of long-term stability of device performance [13] have all been investigated. Nevertheless, the photoanode has been regarded as paramount since the factors for improving efficiency such as presenting a high density of light-harvesting molecules, fast electron injection from dyes to networks, fast electron transport within networks, slow back electron transfer to oxidized dyes and shuttles, and high open-circuit photovoltages (Voc) are determined, at least in part, by the properties of the anode.
Recently, unconventional approaches for enhancing solar cell performance have also been actively demonstrated. In particular, surface plasmon arising from metal nanoparticles (NPs) has been applied to increase the optical absorption and/or photocurrent in a wide range of solar cell configurations, e.g. silicon solar cells [14, 15], organic solar cells [16] and DSCs [17–21]. However, most of the earlier work on surface-plasmon assisted energy conversion in DSCs reported only improved dye absorption or photocurrent, while the obviously improved device performance was not observed [18, 19]. Investigators have designed and tested various plasmonic light-trapping geometries for enhancing conversion efficiency of DSCs; however, the interaction and working mechanisms in plasmonic DSCs contained metal nanostructures are dramatically complicated. A detailed description of the kinetics of electrochemical and photoelectrochemical processes in plasmonic DSCs has not been established, especially its relation to the Au NP size. Moreover, simple deposition of NPs on the surface of semiconductors is usually unstable under reaction conditions, and they tend to migrate and aggregate into larger particles, leading to the loss of the unique properties of the original NPs [22]. This is particularly true for the case of gold NPs dispersed on TiO2 substrate, a composite of great interest because of its synergistic effect in the promotion of oxidation [23, 24] and photochemical reactions.
In this study, Au NPs with different particle size were synthesized and immobilized on fluorine-doped SnO2 (FTO) substrates. The porous TiO2 films were fabricated on Au NP-FTO using P25 TiO2 powders and by the screen-printing method. The size effects of Au NPs on the PV performance of the formed DSCs were investigated systematically. The photoelectrochemical properties of the plasmonic DSCs were analysed by electrochemical impedance spectroscopy (EIS). The optimal conditions for the fabrication of DSCs with Au NPs modified FTO electrodes were evaluated and explored. An overall conversion efficiency of 6.69% was obtained when the size of Au NPs was around 25 nm, which had a 15% improvement over the performance (5.84%) of bare FTO-based device. The related PV performance enhancement mechanisms and surface-plasmon resonances in DSCs with Au nanostructures are analysed and discussed.
2. Experimental
Au NPs were prepared according to the methods published previously with slight modification [25, 26]. A 381 µl portion of hydrogen tetrachloroaurate (III) (HAuCl4 · 3H2O) aqueous solution (0.1M) was added into 150 ml DI water and brought to a rolling boil with stirring, and then 2 ml of 2% (wt%) trisodium citrate dehydrate solution was added. The solution turned dark blue in about 25 s, and changed to rose red after 70 s, indicating the formation of monodisperse spherical particles. After continuous boiling for another 5 min, the reduction of gold chloride was almost completed. The solution was removed from the heat source and allowed to cool naturally and sit overnight. The Au particle size was around 15 nm. The obtained solution was used as seed solution for the larger size Au NPs' preparation and named as G15. To prepare larger size Au NPs, 40 µl HAuCl4 · 3H2O solution, 24.9 µl hydroquinone and G15 solution with different volume were mixed in 50 ml DI water at room temperature under vigorous stirring over 30 min. The particle size grew up as the volume of G15 solution decreased. The obtained colloidal gold solutions with particle size around 25 nm, 40 nm, 60 nm and 80 nm were named as G25, G40, G60 and G80, respectively.
After cleaning ultrasonically in ethanol and acetone, the fluorine-doped SnO2 (FTO) substrates were immersed into the 3-aminopropyl trimethoxysilane (APTMS, 10% volume ratio) solution for more than 24 h. Then the substrates were soaked in differently sized Au NP suspensions for another 24 h after washing with DI water. At this point, the monolayer of Au NPs with different particle size was formed on the FTO surface and the substrates were ready to use. The TiO2 paste preparation followed the method reported in our previous work [9, 27]. The TiO2 working electrodes were prepared using the prepared TiO2 paste on Au NP-FTO samples by screen printing. The thickness of the porous TiO2 film was controlled by the printing times. The screen-printed layer was annealed at 500 °C in air for 30 min. The TiO2 electrodes were immersed into the dye solution (0.5mM N719 (Solaronix) in acetonitrile and tert-butyl alcohol (volume ratio of 1 : 1)) at room temperature for 20 h after cooling down to 80 °C. The thickness of the porous TiO2 layer was about 10 µm.
After rinsing with acetonitrile, the TiO2 anodes were assembled with the prepared Pt counter electrodes (CEs). The platinum CEs were prepared by the screen-printed technique reported in our previous work [9, 27]. The paste was prepared by mixing H2PtCl6 in a mixture of terpineol and ethyl cellulose. The printed layers were heated at 400 °C for 15 min. The cells were sealed with Surlyn 1702 (Dupont) gasket with a thickness of 25 µm. A drop of electrolyte solution (0.1M Guanidine Thiocyanate (GuSCN), 0.03M I2, 1.0M 1-methyl-3-propylimidazolium iodide (MPII) and 0.5M tert-buthylpyridine in acetonitrile) was introduced into the cell by capillarity. Finally, the holes were sealed using the same Surlyn film and a cover glass with a thickness of 0.7 mm.
The morphologies of the Au NPs deposited on FTO surface were characterized by field emission scanning electron microscope (FE-SEM, JSM-7001F, JEOL). EIS measurements of the DSCs produced by different anodes were recorded with a potentiostat/falvanostat (PG30.FRA2, Autolab) under illumination 100 mW cm−2. The frequency range was from 0.1 Hz to 100 kHz. The applied bias voltage and ac amplitude were set at open-circuit voltage of the DSCs and 10 mV between the CE and working electrode, respectively. The UV–vis absorption spectra of Au NPs suspensions were detected using a UV–vis spectrophotometer (Hitachi U-3900). Photocurrent–voltage measurement was performed with a Keithley model 2440 Source Meter and a Newport solar simulator system (equipped with a 1 kW xenon arc lamp, Oriel) at one sun (AM1.5, 100 mW cm−2). External quantum efficiency (EQE) was taken with an Oriel 300 W xenon arc lamp and a lock-in amplifier M70104 (Oriel) under monochromator illumination, which was calibrated with a monocrystalline silicon diode. The active area of TiO2 electrode was 0.196 cm2.
3. Result and discussion
Figure 1 showed the absorbance of the various prepared Au NPs suspensions. To obviate the concentration differences, the spectrum was normalized between 0 and 1. As shown in the figure, the absorbance peak red shifted from 519 to 579 nm along with the size growth of the Au particle from 15 to 80 nm. And the peaks became wider due to the broader size distribution in the larger size Au NPs suspensions.
Figure 1. Absorbance spectra of Au suspensions with different particle sizes.
Download figure:
Standard imageFigure 2 showed FE-SEM images of differently sized Au NPs deposited on the FTO surface. The particle size is around 15 nm, 40 nm and 60 nm as shown in figures 2(a), (c) and (e), respectively. Figures 2(b), (d) and (f) are higher magnification images of these particles. When the particle size was smaller than 60 nm, Au NPs were dispersed homogeneously and tightly attached to the FTO surface without aggregation, as shown in figures 2(b) and (d). The irregular and rough crystalline structures under the Au NPs were due to the fluorine-doped SnO2 layer coated on the glass, and Au NPs seemed mainly distributed and aggregated near the gaps between SnO2 particles on the surface of FTO. But, when the size of the particle was up to 60 nm, NPs aggregated together and formed randomly distributed NP islands as shown in figure 2(e). These Au NPs or islands are useful for investigating surface-enhanced Raman spectroscopy and other plasmonic phenomena [28, 29]. SEM characterizations of Au modified anodes after the whole DSC device fabrication and test process were carried out. TiO2 layer was removed from the FTO layer on the glass substrate by disassembling the device and soaking the unpacked anode in acetone/water solution and with sonication. SEM images of the resulted FTO (facing TiO2) and peeled TiO2 (facing FTO) layers are shown in figures 2(g) and (h), respectively. The small white spots, grey aggregates and big structures correspond to Au NPs, TiO2 and FTO crystals in figure 2(g), respectively. Au NPs could drop into the ultrasonic bath during the sonication treatment, but some Au NPs were still found to be dispersed on the FTO layer, and their sizes and forms did not change after 500 °C annealing or due to the corrosion caused by iodide-based electrolyte, as can be seen from figure 2(g). On the other side, Au NPs were also found on the surface of the peeled TiO2 film in figure 2(h). Based on our observation, Au NPs can keep its particle form under relatively good conditions even after 500 °C annealing.
Figure 2. SEM images of differently sized Au NPs deposited on FTO surface. (a) (c) (e) Low magnification, (b) (d) (f) high magnification, (g) peeled FTO layer (facing TiO2), (h) peeled TiO2 layer (facing FTO).
Download figure:
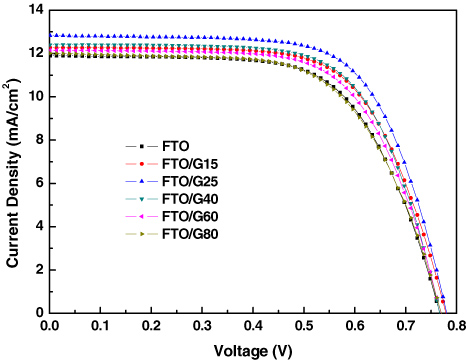
Standard imageFigure 3 showed the photocurrent density–voltage (J–V) curves of the DSCs. The PV parameters were listed in table 1. The performance of DSCs fabricated with anodes deposited on bare FTO substrates was provided for comparison in the table. As can be seen from table 1, the performance, especially the photocurrent, of DSCs containing Au NPs was significantly affected by the particle size of Au NPs. The PV performance improved with increasing size of Au NPs from 15 to 25 nm, and then decreased when the particles continually grew bigger. As the size of Au NPs was up to 80 nm, the energy conversion in the cell based on G80 was smaller than that with bare FTO substrate. From table 1, the best performance was achieved using the anodes prepared on G25-FTO. The best cell showed a short-circuit current density (Jsc) of 12.84 mA cm−2, open-circuit voltage (Voc) of 0.78 V and fill factor (FF) of 66.74%, yielding the highest efficiency (η) of 6.69%. The cell exhibited a 15% improvement over the performance (5.84%) of bare FTO-based device.
Figure 3. Photocurrent–voltage (I–V) characteristics of DSCs fabricated with different anodes.
Download figure:
Standard imageTable 1. Performance characteristics of DSCs fabricated with different anodes under 100 mW cm−2.
| Sample | Jsc (mA cm−2) | Voc (V) | FF (%) | η (%) |
|---|---|---|---|---|
| FTO | 11.90 | 0.77 | 63.99 | 5.84 |
| FTO/G15 | 12.26 | 0.78 | 65.65 | 6.27 |
| FTO/G25 | 12.84 | 0.78 | 66.74 | 6.69 |
| FTO/G40 | 12.41 | 0.77 | 66.55 | 6.33 |
| FTO/G60 | 12.21 | 0.77 | 65.25 | 6.10 |
| FTO/G80 | 12.10 | 0.77 | 62.42 | 5.78 |
From table 1, the averaged data likewise showed gold NP (15–60 nm in size) containing cells to be superior to gold-free cells. The efficiency enhancement in the presence of gold can be seen to arise chiefly from an increase in photocurrent density. The Voc values are almost the same. From FE-SEM pictures shown in figure 2, the per cent coverage area of both Au NPs and islands was small. When a small quantity of metal NPs or islands was induced into the TiO2 anodes, the Voc of the cell was mainly determined by the redox potential and the Fermi energy of the TiO2, independent of the Fermi energy of the metal [30]. Thus, the result of PV measurement is consistent with the FE-SEM measurement and analysis. From figure 3, the overall conversion efficiency of a DSC is improved when Au particles with a size smaller than 80 nm are added. Compared with pure TiO2 films, the mixed films containing Au particles with a size of 15–60 nm exhibit an increase in the power-conversion efficiency from 4% to 15%. Ding et al once reported that DSCs based on anodes containing 22 vol% SiO2 spheres coated with Ag particles exhibited an increase in the power-conversion efficiency from 2.7% to 4.0% [20]. Better results were obtained using our proposed method.
EQE is more pertinent than photocurrent–voltage measurement for studying photocurrent generation of DSCs. It is a product of light-harvesting efficiency, electron injection efficiency from excited dye molecules to TiO2 NPs, and electron collection efficiency at the anode. In order to emphasize the positive contribution of Au NPs to the photocurrent, the EQE data of Au modified DSCs were normalized by dividing that of non-Au modified DSC. Figure 4 shows the normalized EQE curves of DSCs using anodes containing Au NPs with different sizes. As the Au particle was smaller than 60 nm in size, EQE of the DSC with these Au NPs is obviously improved in the 500–700 nm wavelength region when compared with that of the device based on the pure TiO2 film. EQE of the cell using 25 nm Au NPs is most significantly improved. In this device, the EQE enhancement maximum (1.28) is at 535 nm, which is very close to the absorption peak position of 25 nm Au NPs shown in figure 1. In Au NPs incorporated anodes, due to the peaking absorption of Au NPs from 519 to 579 nm shown in figure 1, sunlight can effectively couple with plasmon absorption in this range. Consequently, dye molecules placed in the vicinity of Au NPs usually absorb more photons and resulted in EQE enhancement in this regime. From figure 4, the trend in EQE enhancement for different Au particle sizes is consisted with that from I–V measurement and analysis shown in figure 3.
Figure 4. Normalized EQE curves of DSCs using TiO2 anodes containing Au NPs with different sizes.
Download figure:
Standard imageAs well known, plasmon-enhanced energy conversion in DSCs can be associated with local field enhancement effects. Localized surface-plasmon resonance (LSPR) of individual metal particles and various particle structure configurations allows ways for the realization of strong-field confinement and enhancement [31–34]. The optical response, LSPRs and local field distributions are strongly dependent on the particle shape and size, the interaction between particles, and the polarization of the incident light. We consider the amplitude of the electric vector of the incident plane wave is normalized to unity, and the incident wave (wavelength λ = 530 nm) propagates along the z-coordinate, electric vector is directed along the x-coordinate, and magnetic vector along the y-coordinate, in the spherical coordinate system with the origin, situated at the sphere centre. The dielectric function of gold containing the size effect of surface scattering is considered from our previous work [35]. Considering that the refractive index of the FTO layer is n = 1.74 and TiO2 particles have a refractive index
 (anatase) [36], the dielectric constant (εM) of the medium surrounding the sphere is assumed as 2.122. Figure 5 shows the internal and external intensities along the z-axis as a function of r/a for Au spheres with different diameters (2a) based on the Mie theory [37, 38]. Mathematica 4 was used [39]. For comparison study, the internal and external intensity of a transparent sphere with a size parameter (2πa/λ) of 20 and an index of refraction of 1.5 is calculated and shown in the inset of figure 5. The figure of this transparent sphere shows that for r/a < −1, outside the illumination surface of the sphere, the total external intensity oscillates due to the interference between the incident and backscattered waves. For −1 < r/a < +1, the internal intensity is highly oscillatory as a result of interference between the refracted and internally reflected components. For r/a > +1, the intensity rises to a focal peak outside the shadow side of the sphere. This calculation curve is completely consistent with figure 4.32 in [40]. The intensity distribution of a gold sphere is considerably different from that of the transparent sphere shown in the inset of figure 5. For an Au sphere, there are significant electrical field enhancements in regions around the sphere but not outside the shadow side of the sphere. As the size of the sphere is increased from 5 to 15 nm, the maximum intensity enhancement in calculations is increased from about 6 to 8. The maximum enhancement factor for a 25 nm sized sphere is very close to 8. But, further increasing the size of the sphere from 25 to 80 nm, the maximum intensity enhancement is decreased from about 8 to 4. By comparing the results shown in table 1 and figure 5, the photocurrent density of DSC containing Au NPs almost follows the same trend as the calculated maximum intensity enhancement factor with the change in the size of Au NPs. The calculated maximum intensity enhancement factor for 15 nm sized spheres is a little higher than that for 25 nm sized ones; however, from figure 1, SPR band for G25-Au NPs is at around 530 nm, where the absorption maximum of the sensitizer (N719) is located. As a result, the cell containing G25-Au NPs exhibited the maximal improvement.
(anatase) [36], the dielectric constant (εM) of the medium surrounding the sphere is assumed as 2.122. Figure 5 shows the internal and external intensities along the z-axis as a function of r/a for Au spheres with different diameters (2a) based on the Mie theory [37, 38]. Mathematica 4 was used [39]. For comparison study, the internal and external intensity of a transparent sphere with a size parameter (2πa/λ) of 20 and an index of refraction of 1.5 is calculated and shown in the inset of figure 5. The figure of this transparent sphere shows that for r/a < −1, outside the illumination surface of the sphere, the total external intensity oscillates due to the interference between the incident and backscattered waves. For −1 < r/a < +1, the internal intensity is highly oscillatory as a result of interference between the refracted and internally reflected components. For r/a > +1, the intensity rises to a focal peak outside the shadow side of the sphere. This calculation curve is completely consistent with figure 4.32 in [40]. The intensity distribution of a gold sphere is considerably different from that of the transparent sphere shown in the inset of figure 5. For an Au sphere, there are significant electrical field enhancements in regions around the sphere but not outside the shadow side of the sphere. As the size of the sphere is increased from 5 to 15 nm, the maximum intensity enhancement in calculations is increased from about 6 to 8. The maximum enhancement factor for a 25 nm sized sphere is very close to 8. But, further increasing the size of the sphere from 25 to 80 nm, the maximum intensity enhancement is decreased from about 8 to 4. By comparing the results shown in table 1 and figure 5, the photocurrent density of DSC containing Au NPs almost follows the same trend as the calculated maximum intensity enhancement factor with the change in the size of Au NPs. The calculated maximum intensity enhancement factor for 15 nm sized spheres is a little higher than that for 25 nm sized ones; however, from figure 1, SPR band for G25-Au NPs is at around 530 nm, where the absorption maximum of the sensitizer (N719) is located. As a result, the cell containing G25-Au NPs exhibited the maximal improvement.
Figure 5. Internal and external intensity along the z-axis as a function of r/a for Au spheres with different diameters (5–80 nm) and a sphere with a size parameter of 20 and an index of refraction of 1.5 (inset).
Download figure:
Standard imageTo study the kinetics of electrochemical and photoelectrochemical processes including the elucidation of the electronic and ionic transport processes in plasmonic DSCs contained Au NPs, EIS measurements of cells were carried out. There are many charge-transfer processes in energy conversion in a DSC and these processes interact with each other in a complicated manner. The EIS investigation of the DSCs provides valuable information for the understanding of PV parameters [41–44]. Generally, all the EIS spectrum of DSCs containing liquid electrolyte exhibits three semicircles in the Nyquist plot or three characteristic frequency peaks in the Bode phase plot. The resistance Rct1, Rct2 and Zw are assigned to redox charge transfer at the CE, electron transfer at the TiO2/dye/electrolyte interface and the Warburg diffusion in the electrolyte in the order of decreasing frequency. The ohmic resistance (Rs) is mainly due to the sheet resistance of FTO [45, 46].
Figure 6 showed the impedance spectra of DSCs associated with different anodes. The equivalent circuit was shown in the inset of figure 6(a). From the Nyquist plot (figure 6(a)), all the fabricated DSCs had quite similar Rct1 values because they were prepared using the same CEs. It was found that there was a distinct difference in Rct2 among DSCs with different anodes. Compared with the bare FTO-based DSCs, the introduction of the Au NPs enlarged the Rct2 value as shown in figure 6(a). The Rct2 value increased with the increase in the size of Au NPs.
Figure 6. Electrochemical impedance spectra of DSCs fabricated with different anodes. (a) The Nyquist plot. (b) The Bode phase plot. The equivalent circuit of this work is shown in the inset of (a).
Download figure:
Standard imageAs well known, gold has long been regarded as being catalytically far less active than other transition metals. However, gold NPs dispersed on metal-oxide supports are active catalysts for a variety of chemical reactions [47, 48]. It has been demonstrated that the unique catalytic activity strongly depends on the type of metal-oxide support, the preparation method, and particularly the size of the Au clusters. When gold is deposited on selected metal oxides as hemispherical ultra-fine particles with diameters smaller than 5 nm, it exhibits surprisingly high activities and/or selectivities in the combustion of CO and saturated hydrocarbons, the oxidation–decomposition of amines, the reduction of nitrogen oxides, etc [48]. In our work, when Au NP was small, the catalytic effect of Au NPs sited on TiO2 support could increase the reaction rate between the TiO2/dye and the redox electrolyte in DSCs, thereby reducing the corresponding electron transport resistance. Conversely, as the gold size became larger, Au NPs easily aggregated and formed islands as shown in figure 2, which could not preferentially attach to specific sites on the TiO2 surface, resulting in the decrease of catalytic activity. Thus, the Rct2 value increased along with the growth of Au particle size. In the conventional DSCs, the dyes absorb incident light and generate electrons in excited states, which inject into the TiO2 NPs. The oxidized dye molecules are regenerated by electrons transferred from iodide. The regenerative cycle is completed by reducing triiodide to iodide at the Pt cathode. The electrons in TiO2 diffuse to the current collector (FTO). In the plasmon-enhanced DSCs using Au NPs, there is direct contact between Au and the electrolyte and the surface of Au NPs serves as a recombination site for the photogenerated electrons and triiodide ions, resulting in the increase in Rct2 by comparison with that in the bare FTO-based DSCs shown in table 1. As the introduction of Au NPs enlarged the Rct2 value compared with the case using the bare FTO, one would expect a higher Jsc value in the latter. However, the enhanced local electromagnetic fields produced by the plasmonic Au NPs with a size smaller than 80 nm coupled light very efficiently from the far-field to the near-field of the dye molecule monolayer, resulting in the enhancement the optical absorption of the dye and the increase of Jsc and thus η values compared with those using the bare FTO. Nevertheless, when the Au particle is large up to 80 nm, the reduced electromagnetic field enhancement as shown in figure 5 could not overcome the increase in Rct2, the efficiency of the plasmonic DSC became lower than that based on the bare FTO as shown in table 1 and figure 3.
Figure 6(b) showed the corresponding Bode phase plot of the DSCs fabricated with different substrates. The Bode phase diagram contains three characteristic frequency peaks. These three bands are assigned in the order of increasing frequency, to Nernst diffusion impedance of the redox species in the electrolyte, diffusion and recombination of electrons in TiO2 and at TiO2/FTO interface, and charge-transfer process at CE/electrolyte interface. Compared with the DSCs fabricated with bare FTO-based anodes, the characteristic peak based on Au NP-FTO substrates shifted to lower frequency in the intermediate frequency regime. The characteristic frequency can be related to as the inverse of the recombination lifetime (τr), or electron lifetime (τe) in TiO2 film [49–51]. Shibata et al [52] and Kiyonaga et al [53] had reported that the easy electron transfers from TiO2 to Au NPs when small-sized Au NPs contact with TiO2 to form a nanoscale heterointerface. This implies that the presence of small Au NPs provides efficient electron-transfer route with enhanced charge collection due to the connectivity improvement between the TiO2 network and FTO substrates, which contributes to the enhanced Jsc [54].
The LSPR feature of Au NPs is mainly responsible for the increase in Jsc and thus the raise of η in DSC containing Au NPs [17–20]. Along with the increase in the Au NP size, the density of traps on the Au surface increased, and further increased the recombination rate of the photogenerated carriers in the cell. On the other hand, the TiO2 conduction band at the TiO2/Au interface would fluctuate in response to the induction of Au NPs, which may retard the electrons migrating from the surface to the interior of the TiO2 film. As a result, the back-reaction and the dark-reaction might be enhanced because the generated carriers on the conduction band of TiO2 were captured by the dye and the redox in the electrolyte, respectively [30]. Moreover, the large Au NPs agglomerated and formed islands structures which would prevent the incident light from reaching the TiO2 NPs, resulting in the decrease in the photocurrent density. Finally, the catalytic effect of small Au NPs might increase the reaction rate between the TiO2/dye and the redox electrolyte, and thus raise the photocurrent. The combined effects of these factors resulted in the dependence of the performance of the plasmonic DSC on the Au particle size.
4. Conclusions
Plasmon-enhanced energy conversion in DSCs with gold NPs modified FTO electrodes has been investigated. Au NPs with different sizes were grown and immobilized onto FTO glass substrates. The photoelectrochemical properties of the formed DSCs were evaluated in terms of electrochemical impedance spectra and photovoltaic characteristics. The performance of the DSC highly depended on the Au particle size. The Jsc and η increased with increasing size of Au NPs from 15 to 25 nm, and then decreased when the particle increased further. As the size of Au NPs was up to 80 nm, the energy conversion in the cell was smaller than that with bare FTO substrate. Absorbance and EIS data and analyses as well as theoretically calculated surface-plasmon resonances of Au NPs supported the result of the conversion efficiency of the fabricated DSCs. When 25 nm sized Au NPs were used in the photoanode, the conversion efficiency showed the best result. The DSC based on N719 dye yielded a conversion efficiency of 6.69%, representing a 15% improvement over the performance of otherwise identical solar cells lacking Au NPs.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Nos 11274119, 61275038).