Abstract
By exploiting phase-separation in oxide materials, we present a simple and potentially low-cost approach to create exceptional superhydrophobicity in thin-film based coatings. By selecting the TiO2–Cu2O system and depositing through magnetron sputtering onto single crystal and metal templates, we demonstrate growth of nanostructured, chemically phase-segregated composite films. These coatings, after appropriate chemical surface modification, demonstrate a robust, non-wetting Cassie–Baxter state and yield an exceptional superhydrophobic performance, with water droplet contact angles reaching to ∼172° and sliding angles <1°. As an added benefit, despite the photo-active nature of TiO2, the chemically coated composite film surfaces display UV stability and retain superhydrophobic attributes even after exposure to UV (275 nm) radiation for an extended period of time. The present approach could benefit a variety of outdoor applications of superhydrophobic coatings, especially for those where exposure to extreme atmospheric conditions is required.
Export citation and abstract BibTeX RIS
1. Introduction
Non-wetting surfaces offer potentially broad technological benefits, with applications ranging from energy to health. Many synthetic superhydrophobic surfaces (surfaces with water droplet contact angles (CA) exceeding 150°) previously have been engineered to mimic the water repellency of natural living organisms, e.g., exemplified by the leaves of the lotus plant [1–3]. Central to many of these biological systems is the existence of micro- and nano-scale surface structures that generates a composite air–solid interface by trapping a thin layer of air within the surface; this hierarchical topography, coupled with low interfacial surface-energy chemistry, renders the surface superhydrophobic [1, 4–7]. Such bio-inspired, water-repellent surfaces offer significant promise for numerous uses ranging from underwater applications (e.g., anti-biofouling, reduced friction), to anti-condensation (e.g., ice and corrosion mitigation), to surfaces utilized in patterned devices (e.g., complex microfluidic devices) or those requiring reduced maintenance and cleaning [8].
Utilizing a variety of synthesis approaches aimed towards superhydrophobic surfaces with an appropriate roughness or topography, researchers have produced artificial materials ranging from polymers [1, 3, 4] to metals [1, 9–12] to metal-oxides (such as Y2O3 [13], CuO [14], Cu2O [15], Co3O4 [16], ZnO [17, 18], TiO2 [3, 19, 20], V2O5 [21]). Examples of such techniques include template assisted fabrication, crystal growth, electrochemical deposition, differential etching, lithographic techniques, colloidal assembly of particles and phase separation in multicomponent material systems [1, 3]. While all these methods have shown substantial influence on the wetting properties of the surfaces, most are not compatible with large-scale manufacturing, often requiring complicated, time-consuming multi-step fabrication schemes, and specialized equipment/material employment that are not cost-effective. Development of simple, low-cost, scalable, and eco-friendly processing methods and formulations can make a significant impact on the wide-spread utilization of water-repellent surfaces in various fields. It is also important to pursue methods that enable better control of the structure density, morphology, and dimensions for tailored performance on a variety of substrates. Furthermore, it is highly desirable that the topographic surface provide long-term functionality against exposure to various environmental conditions (e.g., UV radiation, temperature). Of the techniques cited above, the last—phase separation method—is a practical way to achieve these objectives: the method allows cost-effective and relatively simple manufacturing formulations for creation of robust superhydrophobic materials in the form of a coating, the application of which is not generally limited by the underlying template. Most previous efforts to construct such surfaces are based on phase separation of polymeric materials in mixed chemical solvent systems [1, 3, 22].
In this work, we describe a simple, one-step synthesis of hierarchical nanocomposite ceramic film surfaces on single crystal and metal templates. The technique exploits the control of phase separation in the metal-oxide TiO2–Cu2O system during physical vapor deposition by magnetron sputtering from a single, composite target. The resulting surface exhibits exceptional superhydrophobic properties after a simple treatment with hydrophobic functional groups. Work is focused on the TiO2–Cu2O binary oxide system because it exhibits complete thermodynamic phase insolubility. This fabrication method does not require complicated and harsh chemical treatment procedures (which may degrade the physical properties of the underlying substrate materials), relies only on industry standard equipment, and is inherently scalable. The coatings that are achieved on various templates and irregular surfaces utilize inorganic materials that are non-toxic, naturally abundant and inexpensive. The chemical, thermodynamic, and environmental stability of the present nanostructured ceramic films merit their practical application as protective functional coatings on surfaces, in particular on metals that are exposed to highly humid environments.
2. Experimental details
2.1. Fabrication of nanostructured composite films
Radio-frequency magnetron sputtering was used to deposit the composite TiO2:Cu2O thin films (thickness = 0.5 μm − 1 μm) onto both single crystal (00l) SrTiO3 (STO) substrates and biaxially textured Cu foils (50 μm-thick) [23] at temperatures ranging from 580 °C to 650 °C. The sputter target was made from a mixture of 50 mol% Cu2O (99.9%, Alfa Aesar) and 50 mol% TiO2 (99.9%, AlfaAesar) powders, prepared by stirring in an automated ball mill for over 24 h to ensure a homogeneous mixing, which were then packed into a 2 inch diameter copper tray. The depositions were conducted in a reducing sputter gas mixture of 96% Ar + 4% H2 (forming gas) at a chamber pressure of 5 mTorr. Forming gas was employed in part to suppress the oxidation of the Cu substrate surface and in part to disrupt the thermodynamic stability of Cu2O, which otherwise results in self-assembled, highly ordered epitaxial nanostructured arrays of TiO2:Cu2O, having vertically aligned interfaces [24]. Rather, the films comprise a random coral-like nanostructured matrix as described below. To create a superhydrophobic surface, the as-deposited films are soaked in a mixture of hexane and 0.5 vol% 1H,1H,2H,2H-perfluorooctyltrichlorosilane (Gelest, 95%) for 15 min and subsequently annealed in air in an oven at 115 °C for 15 min
2.2. Characterization
The phase identity and crystalline structure of the films were characterized by a PANalytical Empyrean series-2 x-ray diffractometer. Plan-view surface morphology of the composite films was examined using a Hitachi S-4100 high-resolution field-emission type scanning electron microscope equipped with an energy dispersive spectroscopy (EDS) unit. Atomic force microscopy (AFM) in the contact mode was employed to examine the nucleation and the surface roughness of the coatings. The surface wettability was evaluated by the sessile drop technique using an Attension Theta model T301 optical tensiometer (Biolin Scientific, Finland). Static CA were determined by taking the average of at least ten 4 μl water droplets dispensed at different positions on the film. Roll-off angles were established by using a manual tilting stage. The UV stability of the superhydrophobic surfaces was evaluated by exposing the samples to a 275 nm light source at an irradiation intensity of 1.8 mW cm−2 for up to 72 h. The CA were recorded after each successive day of exposure.
3. Results and discussion
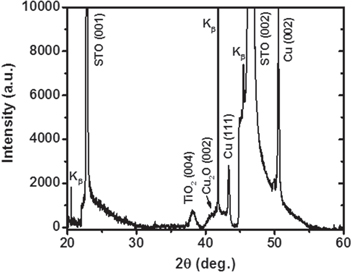
Figure 1 is a typical θ–2θ x-ray diffraction (XRD) pattern of a composite film co-deposited from a single sputter target composed of 50 mol% TiO2 and 50 mol% Cu2O onto a (001) STO single crystal substrate in a reducing atmosphere. Along with the strong substrate reflections, the spectrum displays weaker peaks indexed for anatase TiO2, cubic Cu2O, and Cu phases. Under the present thermodynamic and kinetic growth conditions, while the TiO2 stability is not an issue, the partial transformation of Cu2O into metallic Cu is evident from the presence of (111) and (002) Cu reflections. It is noteworthy that, whether deposited on STO or on Cu foils, the phase separation was observed in all TiO2:Cu2O films as evidenced by the EDS elemental analysis (data not shown here). Here the XRD spectrum is shown only for the STO substrate due to interference of film reflections with the Cu substrate. The broadened lines of the TiO2 and Cu2O phases may be ascribed to small particle size (the Scherrer effect [25]) or possible partial alloying of the two phases (discussed later).
Figure 1. The θ–2θ XRD scan of a mixed phase TiO2–Cu2O composite film sputter-deposited on SrTiO3 substrate, revealing phase separation of TiO2 and Cu2O phases. It is also evident that the conditions employed during the sputter deposition are favorable for partial reduction of Cu2O to metallic Cu phase.
Download figure:
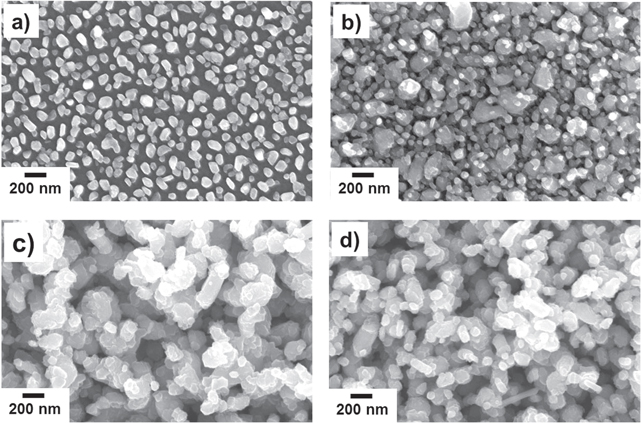
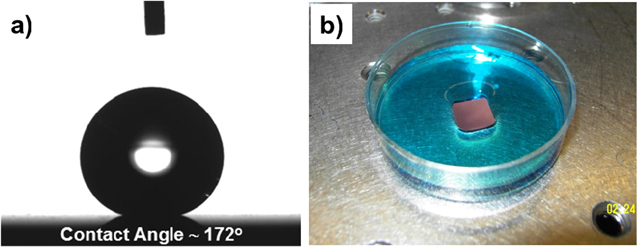
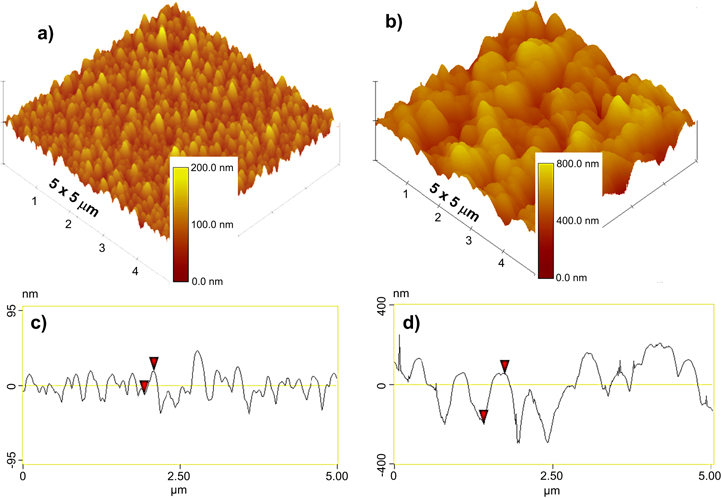
Standard image High-resolution imageConsidering the mechanism governing the phase-separated self-assembly process [26–28], this transformation is imperative for the realization of a random nanostructured film matrix, rather than a self-assembled, vertically ordered nanopillar array, as was observed in our previously published work on the same material system for photovoltaic applications [24]. In general, two broad mechanisms usually dictate phase-separated self-assembly: (i) thermodynamic stability combined with the insolubility between chosen phases, driving the phase separation, and (ii) minimization of the elastic strain (i.e., lattice misfit strain) and interfacial energies, combined with preferential isostructural growth (i.e., growth of each phase onto itself) [27]. In this case however, due to partial transformation of Cu2O into Cu, the large lattice parameter difference of ∼12% between TiO2 and Cu2O is reduced to only ∼4.5% misfit for the TiO2 and Cu, significantly reducing the elastic strain between the two phases. This disruption, in the otherwise energetically favorable isostructural vertical self-assembled nucleation, leads to a highly disordered/random nanostructured surface morphology (figure 2). Shown in figures 2(a)–(c) are the plan-view SEM images of such composite films deposited on STO substrates for various deposition times, illustrating the nucleation and evolution of the growth from a partial to full coverage. Note that the dimensions and surface coverage of the films can easily be controlled by varying the sputter deposition parameters (e.g., time and power). While part of the substrate surface is visible for the film deposited at a short deposition time, a large number of randomly distributed isolated nano-islands with sizes in the range of 50–150 nm are observed (figure 2(a)). With an increase in the deposition time larger grained clusters (∼200–300 nm), assembled by the coalescence of the islands and through stacking of individual phases, are developed (figure 2(b)). Upon further increasing the sputtering time, these clusters eventually progress into a continuous hierarchical morphology with the secondary roughness being constructed with smaller size coalesced individual islands, creating a coral-like appearance (figure 2(c)). Note that by employing the same deposition parameters, similar surface architectures are also attained on textured Cu foils (figure 2(d)). Although both these substrates (STO and Cu) are crystal aligned, we have observed similar coating structures on amorphous glass substrates as well. Such multi-scale surface topographies (figures 2(c) and (d)), after functionalization with low surface-energy organosilane molecules, exhibit exceptional superhydrophobic behavior. This is evidenced by static water droplet CA, θ, reaching to 172° (figure 3(a), data are presented only on STO to avoid redundancy) and sliding angles less than 1°, allowing the water droplets to roll-off very easily, as demonstrated in the supplemental videos SV1 and SV2 (available at stacks.iop.org/NANO/25/245601/mmedia) for the films deposited on STO and Cu platforms, respectively. Consequently, the combination of high static CA and very low sliding angle suggests a very small contact angle hysteresis with essentially no pinning of the water droplet within the structure. This leads to surfaces with an intrinsic water-proof property and self-cleaning ability, implying particular applicability as anti-corrosive protective coatings on metal surfaces. In view of this, figure 3(b) demonstrates so-called 'Moses effect', where the water (blue colored) is expelled from the surface and held back due to its high surface tension (figure 3(b) and supplemental video SV3). Water droplets on such surfaces do not penetrate into the valleys, (i.e., cavities between the bicontinuous film matrix in our case), but rather are suspended on the surface prominences. Note that due to the lack of nanoscale sharpening combined with inadequate coverage, the surfaces presented in figures 2(a) and (b) yielded hydrophobic attributes with CA values of only ∼121° and ∼142°, respectively. It is well established that the wetting properties of a chemically modified solid surface is governed by its morphological structure [1, 4, 5]. Accordingly, for the present case the composition of the TiO2:Cu2O does not have any effect on the hydrophobic characteristics beyond its structure. To provide further insight, as well as to obtain quantitative information about the roughness features, the composite surfaces with two extreme CA performances were analyzed by AFM measurements. Figures 4(a) and (b) show AFM 3D height images for two other samples, made using similar process conditions, revealing clear differences in roughness details. The average surface roughness values (Ra) on a 5 × 5 μm area are significantly increased from ∼17 nm to ∼105 nm with increase in deposition time. Note that as for rougher films, the smaller grains could not be distinguished easily by AFM; essentially, they are being obscured because the scale of the surface roughness is far greater than the size of these features. More detailed 2D roughness profiles illustrate that deposition for the extended period leads to much larger peak heights (figure 4(d)), with much wider peak-to-peak distances (here, peaks represent cluster formations with dimension ranging from 0.5 μm to 1 μm). Additional finer structures at a nano-scale level (observed by SEM image of figure 2(c)) are not observed beneath the larger peaks in AFM due to resolution. Taken together, these analyses can elucidate the observed differences in the wetting properties of the samples. In fact, considering the two distinct limits of wetting characteristics of a rough surface, we can cite the Wenzel [29] and Cassie–Baxter [30] models to correlate the surface roughness with the CA performance. According to the Wenzel theory, water droplets have complete contact with the surface features and fully penetrate into the groves. On the other hand, in the Cassie–Baxter model, air pockets are assumed to be trapped in the cavities between the asperities and become part of the surface, resulting in a composite solid–liquid–air interface where the water droplets ride on the surface protrusions with air pockets acting as a cushion at the film-water interface. A large fraction of trapped air reduces the area of contact between a droplet and the roughened surface, thus preventing penetration of the water into the surface structure. According to the Cassie–Baxter model, the contact angle, θCB, on a composite surface state is given by the sum of weighted average of all the contributions from the different phases (i.e., liquid–solid and liquid–vapor) as: cos θCB = Φs cos θS + Φv cos θv), where Φs and Φv are the area fractions of the solid and vapor on the surface (i.e., Φs + Φv = 1), respectively; and θS is the intrinsic contact angle on a corresponding flat surface having the same surface chemistry. Assuming θv = 180° (i.e., CA of water on the air), the above equation can be written as cos θCB = −1 + Φs (cos θS + 1). Now, considering the two distinct CA performances obtained on samples at hand, the wetting properties of the surfaces shown in figures 2(a) and (c) qualitatively agree with the Wenzel and Cassie–Baxter modes, respectively. According to the typical static CA values obtained on the chemically salinized smooth reference STO or Cu surfaces (i.e., 110°) and on the phase-separated nanostructured coatings (i.e., 165°), Φs is calculated to be ∼0.05. Such a low value of Φs (i.e., air occupies about 95% of the contact area) is consistent with both the SEM and AFM observations and signifies that the droplets contact only on the apex of the film nanostructures, and do not penetrate into the surface cavities, resulting in extreme water-repellent ability of the phase-separated composite coatings.
Figure 2. Scanning electron microscopy images of phase separated composite thin films on SrTiO3 at different deposition times: (a) 15 min, (b) 30 min, (c) 120 min. The surface changes from hydrophobic to superhydrophobic with increased sputtering time. (d) Shows the surface morphology of a coated textured Cu metal foil, displaying similar nanostructured features to those obtained on oxide substrates.
Download figure:
Standard image High-resolution imageFigure 3. (a) The profile of a 4 μl water droplet placed on a TiO2:Cu2O:Cu composite film surface on the SrTiO3 platform, showing a static contact angle of 172° after superhydrophobic functionalization by application of covalently bonded fluorinated organosilane groups. (b) Same superhydrophobic coating demonstrating the so-called 'Moses effect', where the water (blue colored) is repelled from the surface.
Download figure:
Standard image High-resolution imageFigure 4. Atomic force microscopy contact-mode 3D topography images of the same samples as presented in (a) figure 2(a) and (b) figure 2(c). Sharp increase in surface roughness with increased film coverage (notice the magnitude of the scale bars) is indicative of a superhydrophobic transition from a wetted Wenzel state to un-wetted Cassie–Baxter state. (c), (d) Surface profiles on a 5 μm line scan from parts (a) and (b), respectively.
Download figure:
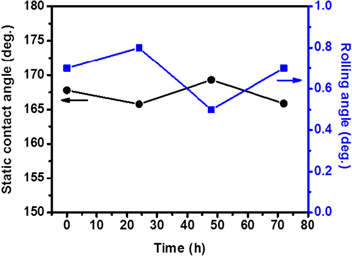
Standard image High-resolution imageIn addition to remarkable water-repellent properties, these nanostructured composite ceramic films also demonstrate excellent resistance to UV-radiation, despite the fact that both TiO2 and Cu2O are employed in solar-driven photocatalysis applications that transform surface properties [31–34]. In particular, TiO2 is widely utilized in areas related to water-splitting and environmental-purification due to its well-known strong ability to reduce/oxidize most organic compounds adsorbed on the surface [35]. After the discovery of UV-induced change of surface wettability (i.e., hydrophobic to superhydrophilic), TiO2 based coatings have also been considered for self-cleaning purposes in various industrial applications [36, 37]. This phenomenon is considered to result from photo generated surface oxygen vacancies which act as preferential sites for dissociative adsorption of water molecules; in turn leading to formation of hydrophilic surface domains [38]. In spite of the photo-catalytic nature of TiO2, the static CA and roll-off performance of the present films, irrespective of the underlying substrate, was found to remain unchanged under UV-exposure at a wavelength of 275 nm for 72 h (figure 5). This indicates that the chemical stability of fluorinated surface groups is not affected. We speculate that a possible reason for this surprising behavior is decreased photocatalytic activity of the TiO2/Cu2O heterojunctions due to a high density of impurity and interfacial defect states with different energy levels (i.e., charge trapping states). These defect states act as recombination centers for photo-generated charge carriers and can therefore significantly limit the catalytic activity. The observed disordered morphology (i.e., roughness), combined with the existence of large elastic strain between the two highly subdivided phases, may result in the high density of trapping states at the heterojunction interfaces that lead to a loss of photo-produced charges. Another possible explanation might be related to the change in band-gap energies and hence the shift in band edge potentials of the composite films if TiO2 is combined with Cu2O as a solid-solution alloy during the deposition process. The degree of this shift could greatly affect the redox ability of the material, as shown in a recent theoretical study predicting that the effective valence band edge energies of solid-solution (TiO2)x –(Cu2O)y alloys shift and become more negative than the redox potential necessary for the generation of the radicals (mainly OH·) that are effective in decomposing most organic compounds [39]. Even though the theoretical conduction band position of these alloys is predicted to be high enough for reduction of oxygen molecules adsorbed on the surface, the predicted unfavorable valence band level would hinder the effective charge separation and hence would suppress the production of radical species; this might explain the maintained superhydrophobic properties of the samples. While detailed investigations are necessary to fully understand the mechanism(s) contributing to the above described phenomenon, it is beyond the intended scope of this study.
Figure 5. Dependence of the static water droplet contact and roll-off angles on exposure time to UV illumination. Samples are phase-separated nanostructured films on a Cu substrate. (Irradiation at a UV light intensity of 1.8 mW cm−2 under ambient conditions in air, 295 K, relative humidity = 60%.)
Download figure:
Standard image High-resolution image4. Conclusions
In summary, we have demonstrated the facile creation of biomimetic metal-oxide composite thin-film surfaces through exploitation of phase-separation in the TiO2–Cu2O system during deposition via magnetron sputtering. These thin film coatings exhibit a multi-scale surface architecture and demonstrate superior superhydrophobicity following a simple, but robust, modification in surface chemistry. The surfaces yield water droplet CA reaching to 172°, with extremely low roll-off characteristics of less than 1°. Moreover, results showed that these coatings provide stability against UV exposure, which may make them attractive for extended outdoor exposure. The overall process technology utilizes industry adopted practices, is potentially scalable, and demands only inexpensive, naturally abundant and eco-friendly inorganic base materials. Because the overall process is not complex, the present approach offers a practical and cost effective basis on which to create a commercially viable new class of leading edge superhydrophobic nanostructured coatings for various applications.
Acknowledgements
This work was supported by the Laboratory Directed Research and Development Program of Oak Ridge National Laboratory, managed by UT-Battelle, LLC for the US Department of Energy. Microstructural property research was conducted at the Center for Nanophase Materials Sciences (CNMS), which are sponsored at Oak Ridge National Laboratory by the Scientific User Facilities Division, Office of BES, US DOE.