Abstract
A series of crystalline shape memory polyurethanes (SMPUs) were synthesized from polycaprolactone diols and 4,4'-methylenedicyclohexyl diisocyanate (H12MDI) with chemical incorporation of allyl isocyanate modified graphene oxide (iGO) into the PU. Actuation of hybrid SMPUs by infrared (IR) absorption of iGO as well as the direct heat actuated SMPUs has been studied in terms of the isothermal crystallization rate, near-IR absorption, and thermal, mechanical, and shape memory properties. It was found that iGO functions as a multifunctional cross-linker at low contents and a nucleating agent at high contents, and as a reinforcing filler, while light absorption by the iGO induced melting of the PU soft segment, giving rise to a shape recovery of over 90% at 1% iGO (G10).
Export citation and abstract BibTeX RIS
1. Introduction
Shape memory polymers (SMPs) respond to external stimuli and their applications are steadily expanding in smart coatings, debondable adhesives, sportswear and textiles as well as conventional packaging materials. Furthermore, potential applications are emerging for biomedical devices and space materials, with the enhancement of the mechanical performance and multiple functionalizations [1, 2]. A number of special issues have been devoted to highlighting the various aspects of this important topic, along with excellent reviews following the recent developments [3–5]. A comprehensive review regarding SMP composites has become available [1].
Mechanical reinforcements and functionalizations of SMP are largely achieved by hybridizations with fillers. Generally, chemical incorporation is superior to physical blending due to the fine dispersion and improved interfaces. Physical and in situ incorporations of layered nanoclay augmented the mechanical strength of the SMPU but decreased the shape recovery [6–8]. Alternatively, when silica nanoparticles were incorporated into the amorphous SMPU via sol–gel reaction, over 99% shape fixity and shape recovery were obtained owing to the cross-linker effect of the particles [9, 10].
SMP can be remotely triggered by an electrical or a magnetic field with a conductive, magnetic filler addition [11]. Currently, two kinds of light-triggered polymeric actuators have been reported, including pure polymers [12] and polymer composites [13, 14]. In each of these cases, the polymeric materials have to be equipped with photoactive functional groups or fillers. That is, actuator materials need to consist of two components on the intra-molecule or inter-molecule level.
Graphene, a one-atom-thick and two-dimensional (2D) single layer, may rival or even surpass carbon nanotubes due to its extraordinary electrical conductivity and mechanical strength [15–18]. Thus, nanocomposites utilizing graphene materials as nanofillers are offering opportunities to impart unprecedented enhancing mechanical properties to polymers at very low loadings [17]. Moreover, the perfect sp2 carbon-network structures of the graphene materials ensure that they have eximious thermal conductivity and IR response [19–21].
SMPs have not fully achieved their technological potential in applications largely because of the limited actuation methods. Huang et al [22] demonstrated that SMPU could be triggered with water. Mohr et al [23] introduced the magnetically induced shape memory effect of composites made from magnetic nanoparticles and thermoplastic SMPs. Leng et al [24] demonstrated that the electrical conductivity of the SMP composite can be improved significantly, which makes it more suitable for Joule heat induced shape recovery. The infrared light possesses wide emission spectra and a unique heating effect achieved in a noncontact manner, therefore actuating SMPs with IR may realize the applications of SMP practically and dramatically [25].
We chemically modified GO using allyl isocyanate (iGO) and incorporated it into the main chain of crystalline PU at various contents. Actuations of the SMPU hybrids by IR absorption as well as the conventional direct heating were studied to evaluate the effects of iGO as light actuators, multifunctional cross-linkers, reinforcing fillers and nucleating agents for the PU soft segments with regard to the SM performance of the hybrids.
2. Experimental details
2.1. Raw materials
Polycaprolactone diol (PCL diol, Mn = 4000) was purchased from Capa (Perstorp Chemicals, Korea). It was dried and degassed at 80 ° C under vacuum for 3 h before use. 4,4'-methylenedicyclohexyl diisocyanate (H12MDI, Aldrich), 2-hydroxyethyl acrylate (HEA, Aldrich), dibutyltin dilaurate (DBTDL, Aldrich), phenyl glyoxylic acid methyl ester (Darocur MBF, Ciba Specialty Chemicals), and allyl isocyanate (Aldrich) were used as received. Graphene oxide was kindly prepared and donated from the lab of Professor Han Mo Jeong, University of Ulsan.
2.2. Chemical modification of GO (iGO)
The process of functionalization of the GO was performed according to the literature procedure [26, 27]. GO (1.2 g) was loaded into a 500 ml round-bottomed flask equipped with a stirrer under nitrogen. Anhydrous DMF (120 ml) was added to create an inhomogeneous suspension. Allyl isocyanate (3.99 g) was then added and the mixture was stirred for three days before the reaction mixture was poured into methylene chloride (2.4 l) to coagulate the product, which was filtered, washed and dried by lyophilization.
2.3. Synthesis of PU/GO chemical hybrids
The overall reaction scheme for synthesizing the composite is shown in scheme 1. PCL diol having a molecular weight of 4000 g mol−1 was first reacted with H12MDI in dimethylformamide (DMF) at 60 ° C for 4 h using a 500 ml four-necked flask equipped with a mechanical stirrer, thermometer and condenser with a drying tube. High molecular weight PCL was employed to make the crystalline soft segment of PU. The NCO terminated PU was end capped with HEA at 55 ° C. This reaction yielded vinyl terminated prepolymer having theoretical molecular weights of about 9000 determined from the [NCO]/[OH] index. The composition of the PCL 4000/H12MDI/HEA was fixed at 2/3/2 (by mol).
Scheme 1. Schematics for synthesizing PU/graphene chemical hybrids.
Download figure:
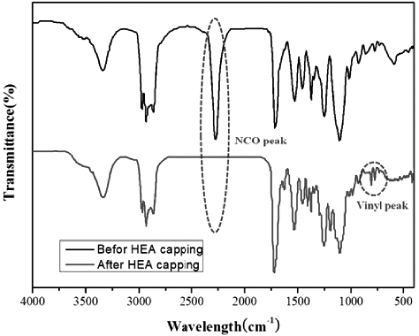
Standard image High-resolution imageFormulations of the reactants are given in table 1. The progress of the reaction was monitored via FT-IR measurements (figure 1), where the absorption peak of NCO at 2270 cm−1 completely disappeared upon capping it with HEA. The HEA capped PU and iGO dissolved in DMF were poured into the flask. A photoinitiator was then added to this dispersion, and stirred for another 2 h before the dispersion was cast on a polyethylene film and partially dried. Then the film was cured using a UV lamp (365 nm, 8W, Crosslink) for 2 h at atmosphere.
Figure 1. FT-IR spectra of the PU prepolymer before and after HEA capping.
Download figure:
Standard image High-resolution imageTable 1. Formulation for preparing the PU/graphene chemical hybrid.
| PCL 4000 (g) | H12MDI (g) | HEA (g) | iGO (wt%) | Tc (° C) | Tm (° C) | ΔT | ΔHm (J g−1) | |
|---|---|---|---|---|---|---|---|---|
| G00 | 26.61 | 2.62 | 0.77 | 0.0 | 16.53 | 46.93 | 30.40 | 46.99 |
| G10 | 1.0 | 14.72 | 45.73 | 31.01 | 38.78 | |||
| G15 | 1.5 | 22.05 | 47.80 | 25.75 | 52.44 | |||
| G20 | 2.0 | 22.93 | 48.44 | 25.51 | 53.72 |
2.4. Measurements
The HEA capping reaction of the H12MDI terminated prepolymer was monitored by FT-IR spectroscopy using the absorption peak of the isocyanate group. ATR-FT-IR (attenuated total reflectance Fourier transform infrared, Thermo Scientific, Nicolet 6500 FT-IR) spectroscopy was used to check the reaction between the vinyl groups of the iGO and HEA capped PU. The NIR/IR spectra were taken in the 1000–3000 nm range using a FT-UV–visible–IR spectrometer, VERTEX 80.
AR-XPS (angle-resolved x-ray photoelectron spectroscopy) and standard XPS measurements were done using a VG-Scientific ESCALAB 250 spectrometer with an Al Kα x-ray source. Thermal analyses were carried out with a differential scanning calorimeter (DSC, Seiko DSC 220) over the temperature range from −30 to 100 ° C at a heating rate of 5 ° C min−1. The state of particle dispersion was studied with the scanning electron microscope (SEM).
For isothermal crystallization the sample was first heated to 80 ° C and kept at that temperature for 20 min to completely erase the thermal history; this was followed by quenching to 23 ° C at which temperature the crystallization was carried out. Isothermal crystallization was done to ensure that the crystallization was completed during the cooling step of cyclic loading and unloading.
The tensile properties of the hybrid were measured at room temperature with a Universal Testing Machine (UTM, Lloyd LRX plus) at a crosshead speed of 500 mm min−1 using specimens prepared according to ASTM D-1822.
The shape memory properties were characterized using a UTM with a heating/cooling chamber attached. The sample was first heated to Tm + 20°C and uniaxially stretched to a maximum strain (εm) of 100%; this was followed by cooling and unloading at Tm −20 ° C. A long enough cooling time was provided to assure crystallization. Upon unloading, a small part of the strain (εm − εu) was instantaneously recovered, leaving an unload strain (εu). Then, the sample was reheated to Tm + 20 °C to recover the strain, leaving a substantial amount of permanent strain (εp). These three steps complete one thermomechanical cycle. Shape fixity (Rf) and shape recovery ratios (Rr) for the cycle are defined as follows [28]:

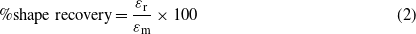
where εr = εm − εp is the recovered strain.
For infrared absorption, an Hg–Xe lamp was used as the light source, positioned 20 cm away from the samples. The power density delivered to the sample was ∼25 mW cm−2 as measured using a light intensity meter. Samples were first elongated to 100% at 65 ° C, and this was followed by cooling to 23 ° C to fix the deformation. The cooling time was typically 15 min. Finally, the deformation was recovered by exposing the sample to the light. In all cases, more than three samples were tested, from which the mean and standard deviation were calculated.
3. Results and discussion
3.1. FT-IR spectroscopy
Figure 1 shows the FT-IR spectra of the PU prepolymer before and after HEA capping. The absorption peak at approximately 2270 cm−1 assigned to the stretching vibration of the NCO group disappeared completely while the vinyl group peak at approximately 750 cm−1 appeared newly after capping the PU prepolymers with HEA.
3.2. ATR-FT-IR spectroscopy
To identify the UV curing reactions between PU and iGO, ATR-FT-IR measurements were carried out and the results are shown in figure 2. The vinyl peaks at 1630 cm−1 (stretching vibration) and 807 cm−1 (twist motion) disappeared upon UV curing, which indicates that the double bonds of PU and iGO are converted into single bonds through the chemical reaction between the two.
Figure 2. FT-IR spectra of the film before and after UV curing.
Download figure:
Standard image High-resolution image3.3. Thermal properties (DSC)
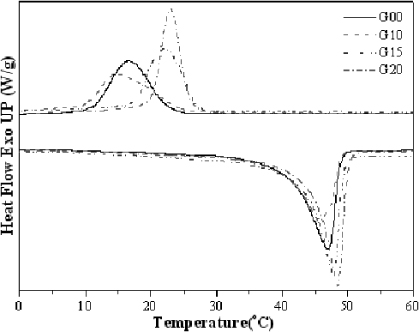
The DSC thermograms of the hybrids are shown in figure 3 along with the crystallization temperature (Tc), melting temperature (Tm), degree of supercooling (ΔT) and heat of melting (ΔHm) data in table 1. It is seen that Tc, Tm and ΔHm for the PU soft segment decrease with 1 wt% iGO (G10) with a slight increase in the degree of supercooling (ΔT), and increase with 1.5 (G15) and 2 wt% (G20) with a decrease in ΔT as compared with the virgin (G00) (figure 3). It seems that the effect of iGO depends on its content. At low iGO contents, iGO acts as effective cross-linker and slows crystallization, while iGO particles act as nucleating agents at high concentration.
Figure 3. DSC thermograms of PU/graphene chemical hybrids.
Download figure:
Standard image High-resolution image3.4. Isothermal crystallization
For the cyclic test, a sample was stretched at Tm + 20 °C and the strain was fixed at Tm − 20 °C. A high degree of crystallization during the cooling step is essential for a high shape fixity ratio. Consequently the crystallization kinetics was studied using the Avrami equation:

where ∅∞ is the equilibrium crystallinity and ∅c is the crystallinity at time t, both being determined from the DSC measurement. The Avrami exponent, n (2.04), and overall rate constant for crystallization, K (5.6 × 10−6), were determined from the Avrami plot prepared using the data for isothermal crystallization at Tm − 20 °C. The half-crystallization time is obtained with  in equation (3):
in equation (3):

Then, using the n and K data determined from the Avrami plot, the half-crystallization time is obtained. The typical half-crystallization time estimated using equation (4) was 4.6 min for G00. This implies that a cooling time over 10 min is necessary for crystallization.
3.5. Mechanical properties
The stress–strain behaviors of the cast films are shown in figure 4. The curves are the averages of five measurements, and detailed values including initial modulus, break strength, and elongation at break are shown in table 2.
Figure 4. Stress–strain behavior of PU/graphene chemical hybrids at 25 ° C.
Download figure:
Standard image High-resolution imageTable 2. Tensile properties of PU/graphene chemical hybrids.
| Series | Young's modulus (MPa) | σb (MPa) | εb (%) |
|---|---|---|---|
| G00 | 106.60 | 19.40 | 713.75 |
| G10 | 163.83 | 23.68 | 673.96 |
| G15 | 185.17 | 24.48 | 633.02 |
| G20 | 119.09 | 18.21 | 868.27 |
The reference PU which was cross-linked with HEA termini shows a typical yield point with elongation at break over 700%. The Young's modulus and strength are increased with G10 and G15 with a small decrease in ductility. However, G20 shows a small increase of Young's modulus and a small decrease of break strength as compared with the virgin PU. The iGO particles seemingly provide nucleating sites but disturb the chain orientation at high concentrations.
3.6. Direct heating shape memory behavior
The typical cyclic loading and unloading behavior of the PU/graphene chemical hybrids operating between Tm + 20 °C and Tm − 20 °C are shown in figure 5 for the first four cycles and the detailed data are given in table 3. The shape fixity is 99% for the first cycle (N = 1) for all samples. With increasing N, G10 gives a small decrease while G15 and G20 give a small increase over the unfilled PU value. The decrease and increase are the same with the crystallinity behavior rather than the modulus. On the other hand, higher shape recovery ratios are maintained for G10 and G15 than for the unfilled case with increasing N, implying that the cross-linking is more pronounced with G10 and G15. It was experimentally found that the shape recovery was insufficient for G20, indicating that cross-linking was insufficient where iGO particles rather disturbed cross-linking reactions between the HEA termini.
Figure 5. Thermomechanical cycles of PU/graphene chemical hybrids.
Download figure:
Standard image High-resolution imageTable 3. Shape fixity and shape recovery of PU/graphene chemical hybrids from direct heating (N: number of thermomechanical cycle).
| G00 | G10 | G15 | G20 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cycle number, N | 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 |
| Shape fixity (%) | 99 | 98 | 97 | 95 | 99 | 98 | 95 | 92 | 99 | 98 | 97 | 96 | 99 | 99 | 98 | 97 |
| Shape recovery (%) | 97 | 92 | 84 | 80 | 99 | 93 | 87 | 85 | 96 | 95 | 94 | 93 | 75 | 67 | 63 | 61 |
3.7. Infrared light-absorption shape recovery
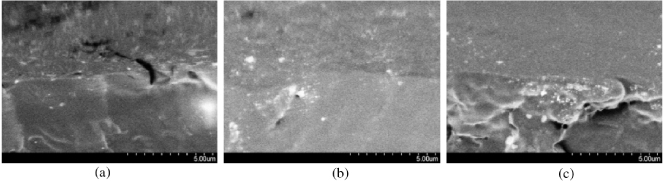
Figure 6 shows that the absorption of near-IR light by PU is significantly enhanced with iGO incorporation. Absorption increases with increasing iGO content, where the effect is more pronounced at low contents due to the fine particle dispersion. The fine particle dispersion at low content is seen from the SEM micrograph in figure 7. However, particle size increases with increasing iGO content.
Figure 6. Near-IR absorption of PU/graphene chemical hybrids.
Download figure:
Standard image High-resolution imageFigure 7. SEM micrographs of PU/graphene chemical hybrids: (a) G00, (b) G15, (c) G20.
Download figure:
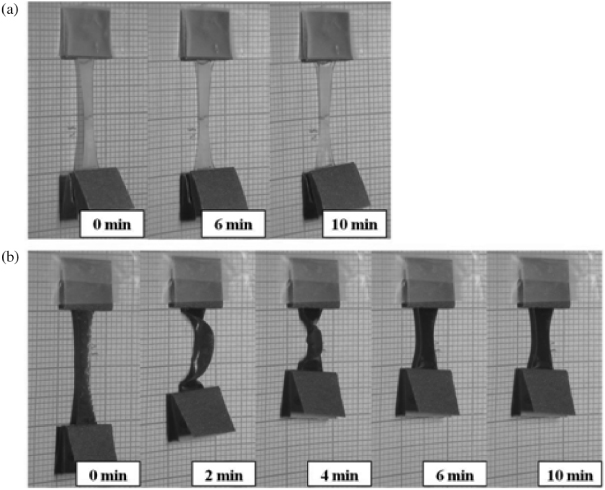
Standard image High-resolution imageFigure 8 shows the typical optical images taken during the shape recovery process based on near-IR absorption. The shape recovery ratio for G00 is about 10% while that for G15 is about 90%, in 10 min.
Figure 8. Optical images of shape recovery versus irradiation time: (a) G00, (b) G15.
Download figure:
Standard image High-resolution imageThe shape recovery ratio versus irradiation time for all the hybrids is shown in figure 9 (table 4). It is obvious that the reference PU (G00) shows insufficient shape recovery upon exposure to the light along with a long induction period of about 4 min. For the hybrids, approximately one minute of induction is noted for all the samples. The initial shape recovery increases with the iGO content, while the equilibrium recovery ratio shows the opposite tendency. It is noted that the equilibrium shape recovery is about 90% for G10. The shape recovery of the semicrystalline polymer is accompanied by a morphology change from crystalline solid to random rubbery state. At the early stage of recovery via light absorption, crystalline mass is being melted and the fast morphology change is due to the melting of the crystalline soft segment of the PU. Consequently, high crystallinity and high ΔHm should provide fast strain recovery. However, at a later stage of recovery, crystalline domains are melted and the morphology change is mainly driven by entropy elasticity where the particles disturb strain recovery. This should give a decrease in equilibrium shape recovery ratio with increase in the iGO content.
Figure 9. Shape recovery ratio versus time for PU/graphene chemical hybrids.
Download figure:
Standard image High-resolution imageTable 4. Shape recovery rate and recovery start time for PU/graphene chemical hybrids for IR absorption.
| Series | Start time (min) | Surrounding temperature (° C) | Sample temperature (° C) | Shape recovery (%) |
|---|---|---|---|---|
| G00 | 6.23 | 44 | 45 | 10 |
| G10 | 1.23 | 51 | 53 | 90 |
| G15 | 1.18 | 49 | 54 | 85 |
| G20 | 0.95 | 51 | 58 | 65 |
4. Conclusions
Shape memory polyurethane (SMPU)/iGO chemical hybrids have been synthesized by UV curing, between the iGO and HEA capped PU. It has been shown that iGO functions as light actuators, multifunctional cross-linkers, nucleating agents and reinforcing fillers, while the relative significance depends on the content. At low content (1.0%) the cross-linker effect is dominant in inducing a high degree of shape recovery, while the nucleating effect is dominant at high content, giving high ΔHm and high shape fixity with repeated cycles. High iGO content disturbed curing reactions and chain orientation, causing decreased break strength of the hybrids below that for the unfilled case.
NIR absorption was significantly increased with the addition and increasing content of iGO, which enabled light induced shape recovery of SMPU which otherwise was not possible. The initial shape recovery ratio increased in proportion to the iGO content owing to the high heat of crystalline melting of the PU soft segment, while the equilibrium recovery ratio showed the opposite tendency due to the disturbance of the strain energy recovery by the large number of particles. Shape recovery over 90% was achieved with 1% iGO through NIR absorption.
Acknowledgment
This study was supported by the National Research Foundation of Korea through the Career Scientist Program of 2012.