Abstract
The excitability of neuronal networks is strongly modulated by changes in pH. The origin of these changes, however, is still under debate. The high complexity of neural systems justifies the use of computational simulation to investigate mechanisms that are possibly involved. Simulated neuronal activity includes non-synaptic epileptiform events (NEA) induced in hippocampal slices perfused with high-K+ and zero-Ca2+, therefore in the absence of the synaptic circuitry. A network of functional units composes the NEA model. Each functional unit represents one interface of neuronal/extracellular space/glial segments. Each interface contains transmembrane ionic transports, such as ionic channels, cotransporters, exchangers and pumps. Neuronal interconnections are mediated by gap-junctions, electric field effects and extracellular ionic fluctuations modulated by extracellular electrodiffusion. Mechanisms investigated are those that change intracellular and extracellular ionic concentrations and are able to affect [H+]. Our simulations suggest that the intense fluctuations in intra and extracellular concentrations of Na+, K+ and Cl− that accompany NEA are able to affect the combined action of the Na+/H+ exchanger (NHE),  /Cl− exchanger (HCE), H+ pump and the catalytic activity of intra and extracellular carbonic anhydrase. Cellular volume changes and extracellular electrodiffusion are responsible for modulating pH.
/Cl− exchanger (HCE), H+ pump and the catalytic activity of intra and extracellular carbonic anhydrase. Cellular volume changes and extracellular electrodiffusion are responsible for modulating pH.
Export citation and abstract BibTeX RIS
1. Introduction
Neonatal seizures are common symptoms of neurological dysfunction being often refractory to anti-epileptic drugs (Rennie and Boylan 2007, Bonifacio et al 2011). In contrast to adults, the administration of anticonvulsants during this early age may increase the frequency and/or severity of seizures due to an intracellular accumulation of Cl− (Pathak et al 2007, Nardou et al 2011). Therefore, the development of new anti-epileptic drugs that effectively improve neonatal seizure control is necessary.
Experimental evidence shows that the excitability of neuronal networks is strongly modulated by changes in pH (Tolner et al 2011). Overall, increases in pH lead to higher excitability, whereas the acidification of the extracellular space has an opposite effect (Chesler and Kaila 1992). These findings have led to the suggestion that the acidification that occurs after intense neuronal activity may act as an intrinsic regulatory mechanism that leads to the cessation of epileptiform events (de Curtis et al 1998, Xiong et al 2000).
The importance of pH control for neuronal network excitability seems to be more prominent in immature brains. Seizures occur more frequently during the neonatal period (Hauser et al 1993) and spontaneous epileptiform activity in hippocampal slices of neonates is highly sensitive to intracellular changes in pH (Xiong et al 2000, Ruusuvuori et al 2010). As an example, a small decrease in intracellular pH induced by the application of weak membrane-permeable acids on CA3 pyramidal neurons leads to a transient blockage of neuronal depolarization. Further, repolarization in these cells occurs at the same time as intracellular pH recovery (Ruusuvuori et al 2010). From investigations performed in neonate brain slices, it has been suggested that the suppression of depolarization caused by weak acids may be due to changes in mitochondrial metabolism processes (Holmgren et al 2010, Bregestovski and Bernard 2012). However, a pH-dependent suppression of depolarization also occurs in standard physiological solutions with 10 mM glucose, even when the weak acid applied is a substrate of mitochondrial ATP production (L-lactate but not D-lactate) (Pavlov et al 2012).
The development of techniques that are capable of interfering with pH requires an appropriate understanding of the mechanisms responsible for intra and extracellular pH changes during epileptic seizures. Xiong et al (2000) and Xiong and Stringer (2000a) investigated dentate gyrus pH changes in hippocampus slices during non-synaptic epileptiform activity (NEA). The authors observed that during the ictal period, a progressive reduction in intracellular pH occurred in neurons as well as in the extracellular space. During NEA, neuronal activity becomes refractory to anti-epileptic drugs that affect synaptic transmission, which can be clearly observed in hippocampus slices bathed with a solution containing zero-Ca2+ and high-K+ (Covolan et al 2014, Miranda et al 2014). In the dentate gyrus, NEA is characterized by an intense ictal period with prolonged extracellular negative dc-shifts superposed with population spikes (Pan and Stringer 1997, Xiong and Stringer 2000b, Almeida et al 2008). Concurrent with field potential changes, when intense neuronal depolarization culminates with the development of high frequency bursts of action potential (Pan and Stringer 1997) and extracellular K+ accumulation (Xion and Stringer 2000). Concomitant changes estimated by computational simulation (Almeida et al 2008) include a decrease of [Na+]o and [Cl−]o from 138.5 to ∼100 mM and from 133.3 to ∼90 mM, respectively.
Changes in the intra and extracellular concentrations of Na+ and Cl− may also influence the pH, which can be directly modulated by H+ transport via H+/Na+ exchanger or the H+ pump. Indirectly, changes in the concentration of  caused by the activation of exchangers, such as
caused by the activation of exchangers, such as  /Cl−, may also affect the pH. Therefore, the intense ionic movement associated with NEA may activate exchangers of H+ and
/Cl−, may also affect the pH. Therefore, the intense ionic movement associated with NEA may activate exchangers of H+ and  and, consequently, contribute to pH changes.
and, consequently, contribute to pH changes.
Computational models are being used for studying non-excitable (Hernández and Cristina 1998, Bodenstein et al 2010) and excitable cells (Almeida et al 2004, Florence et al 2009). Overall, they comprise a useful strategy to understand the dynamic of very complex systems. For studying epileptiform activity in absence of Ca+2, the model proposed by Almeida et al (2008) allows simulating the NEA and investigating possible mechanisms involved in epileptiform neuronal activity. The inclusion of mechanisms related to pH changes in this model may help guiding future experimental investigations.
In the present work, possible pH-associated mechanisms were incorporated to the NEA model. We found that pH changes may result from the combined action of the NHE, HCE, H+ pump and the catalytic activity of intra and extracellular carbonic anhydrase. The activation of these mechanisms is promoted by intense fluctuations in intra and extracellular concentrations of Na+, K+ and Cl−, which are concomitant changes associated with the intense neuronal activity during ictal periods of the NEA.
2. Methods
Mechanisms responsible for pH changes in the intra and extracellular space were investigated using the previously designed computational model for the non-synaptic epileptiform activity proposed by Almeida et al (2008) and Lopes et al (2013). The investigation was undertaken with sequential inclusion of cell mechanisms related to ionic transportation. After interpreting the simulations, the need for a specific mechanism to control ionic transporters and reactions associated with the changes in H+ and  was analyzed. Our simulations were then compared with experimental data described in the literature.
was analyzed. Our simulations were then compared with experimental data described in the literature.
To provide a clearer appraisal of the model, in the sections below we describe the corresponding actualization of its structure based on new experimental findings and the incorporation of the investigated mechanisms involving pH changes.
2.1. The Nonsynaptic epileptiform activity model
Our model was based on that proposed by Almeida et al (2008) and Lopes et al (2013), designed to study mechanisms underlying the nonsynaptic epileptiform activity in the granule cell layer of the hippocampal dentate gyrus.
In the present work, new elements were introduced into the model and its basic original characteristics were confirmed. As reported in the literature, recordings of epileptiform activity in hippocampal slices of adult naïve rats are not affected by bumetanide, a specific blocker of the cation–chloride cotransporter NKCC (Margineanu and Klitgaard 2006). As a result, the blockage of the NKCC is expected to decrease the neuronal chloride concentration. This finding indicates that the NKCC expressed in brain slices of naïve rats is almost inactive. As NEA does not seem to depend on NKCC, in this current version of the model the onset of the nonsynpatic epileptiform events was not based on NKCC activity.
It has been reported that the KCC cotransporter operates very close to a thermodynamic equilibrium, meaning that it may revert its flux depending on K+ and Cl− chemical gradients (Payne et al 2003). The influx of Cl− induced by extracellular potassium increases is mediated by the KCC. As a result, KCC affinities to Cl− and K+ in the intracellular surface of the neuronal membrane were readjusted.
To accomplish the main objective of the present work, that is, to determine possible mechanisms related pH changes during NEA, the ionic dynamics of H+ and  were incorporated into the model. Mechanisms associated with the control of H+ and
were incorporated into the model. Mechanisms associated with the control of H+ and  concentrations were investigated analyzing correspondent simulations.
concentrations were investigated analyzing correspondent simulations.
All changes performed in the model implicate additional adjustment in parameters. As shown in the supplementary information (SI), these can be divided in steady state parameters or investigative parameters (Almeida et al 2008).
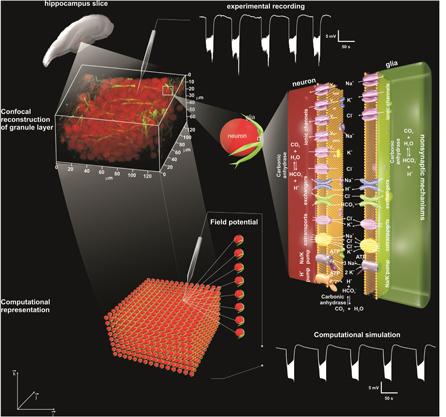
A schematic drawing of the model is shown in figure 1. A tri-dimensional network of functional units represents the dentate gyrus granule cell layer. Functional units were composed of three interconnected compartments: neuronal cell body, glial processes and extracellular space. The communication between each subunit was based on the active and passive ionic transports between the extracellular space, neurons and glial cells. An electrodiffusion process governed the ionic movements through the extracellular space. In this version of the NEA model, the effects of the intracellular electrodiffusion on the intracellular control of the ionic levels have been included.
Figure 1. Diagrammatic representation of the model's design showing its three-dimensional representation and basic mechanisms. Representation of a hippocampal slice, where a portion of the granular layer corresponds the simulated network. When this portion is magnified by confocal microscopy (staining with Neu-N, neurons, and GFAP glial cells), the functional unities (neuron—extracellular space—glial cell segment) can be visualized with a sample of an experimental recording of the extracellular potential of a NEA. The magnified representation of a functional unity shows all the mechanisms used in the simulations of the non-synaptic epileptiform activity and pH changes. Detailed information about the ionic channels, exchangers, cotransporters and pumps, as well as the non-synaptic connections of the network considered in the model are provided in the supplementary information available at stacks.iop.org/PB/12/056007/mmedia. For the present investigation, we highlight the representation of mechanisms related to pH changes: extracellular electrodiffusion of H+ and  CO2 hydration, glial and neuronal
CO2 hydration, glial and neuronal  /Cl− exchanger and neuronal NHE. These mechanisms are circled in the diagram. A network of functional unities was used to simulate the extracellular potential of the NEA and the concomitant changes like ionic concentrations and ionic fluxes (adapted from Lopes et al 2013).
/Cl− exchanger and neuronal NHE. These mechanisms are circled in the diagram. A network of functional unities was used to simulate the extracellular potential of the NEA and the concomitant changes like ionic concentrations and ionic fluxes (adapted from Lopes et al 2013).
Download figure:
Standard image High-resolution imageA brief description of the subcellular mechanisms used to represent the neuronal electrochemistry activity is present below. Details of the equations, parameters, variables and a pseudocode showing how the model was implemented from a computational standpoint are provided in SI.
The Goldman–Hodgkin–Katz equation for current was used to calculate the ionic density fluxes through ionic channels and gap-junctions. The following ionic channels were represented in granule cells: fast gating Na+ channels, persistent Na+ channels, K+ rectifier channels, A-type K+ channels, voltage-dependent Cl− channels and Cl− channels with constant permeability. Ionic channels included in the representation of the glial cells processes were: Na+, K+ and Cl− constant permeability channels and K+ rectifier channels. Permeability changes of the Na+ and K+ voltage dependent channels were based on the Hodgkin–Huxley formalism (Hodgkin and Huxley 1952).
The inward movement of two potassium ions and the extrusion of three sodium ions by the Na/K pump, which is the source of its electrogenic effect, was represented by a fictitious ion A+ flowing according to the electrochemical gradient. The intra and extracellular ionic concentrations of this ion were determined by equations representing the Albers–Post model (Rodrigues et al 2008, 2009).
The representation of the cotransporters was based on the differential equations deduced to represent the chemical reactions of the ionic bindings and the transport through the membrane.
In the previous versions of the model (Almeida et al 2008, Lopes et al 2013), Na+/H+ (NHE) and  /Cl− (HCE) exchangers were only represented as contributing to control the Na+ and Cl− homeostasis. In the present study, these were included to represent the effects of exchangers on concentration changes of H+ and
/Cl− (HCE) exchangers were only represented as contributing to control the Na+ and Cl− homeostasis. In the present study, these were included to represent the effects of exchangers on concentration changes of H+ and  determining pH changes during the NEA. In addition to these exchangers, the H+ pump and the CO2 hydration reaction, which is catalyzed by carbonic anhydrase, were also incorporated. The mathematical representation and the mechanisms involved in pH regulation are described in the results section.
determining pH changes during the NEA. In addition to these exchangers, the H+ pump and the CO2 hydration reaction, which is catalyzed by carbonic anhydrase, were also incorporated. The mathematical representation and the mechanisms involved in pH regulation are described in the results section.
In the absence of the synaptic connections, mechanisms involved in neuronal couplings, the electric field effect and extracellular ionic fluctuations are dependent on gap-junctions (Almeida et al 2008, Volman et al 2011, Lopes et al 2013). As already mentioned, the gap-junction effect was estimated by calculating ionic fluxes using the Goldman–Hodgkin–Katz current equation. The field effect was determined based on the field produced by transmembrane currents assuming a quasi-stationary process. The extracellular resistivity was estimated based on extracellular volume changes, which were also estimated in terms of osmolarity changes. These resistivity changes modulate the ephaptic effect of the extracellular currents on neighboring cells.
As for neuronal coupling mediated by extracellular ionic fluctuations, extracellular ionic changes promoted by a group of cells modified the ionic concentrations of the neighboring regions to modulate activity of these cells.
The equation used to estimate the ionic changes associated with extracellular electrodiffusion was based on the Nernst–Planck equation, in which the ionic movement is caused by the mutual effect of extracellular diffusion and electric field. The electric field had two components. The first was calculated based on the ionic distribution through the extracellular space and constitutes the slow component of the potential, usually named dc shift (Almeida et al 2004). The second is generated by the transmembrane ionic currents (Almeida et al 2008, Lopes et al 2013).
Osmolarity changes generated by the ionic exchange between intra and extracellular spaces promote tissue volume changes. During the ictal period, the intracellular volume increases due to a higher intracellular osmolarity. This is associated with a decrease in extracellular volume (Lopes et al 2013). Cellular volume changes were determined as a function of the osmolarity gradient between intra and extracellular spaces.
2.2. Mechanisms related to pH changes
2.2.1. H+ and  extracellular electrodiffusion
extracellular electrodiffusion
The inclusion of H+ and  was performed considering exclusively electrodiffusion in the extracellular space and the effect of extracellular volume changes on their concentrations:
was performed considering exclusively electrodiffusion in the extracellular space and the effect of extracellular volume changes on their concentrations:
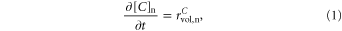

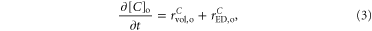
where
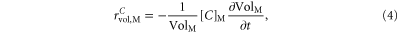
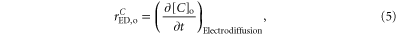
and ![${(\frac{\partial {[C]}_{{\rm{o}}}}{\partial t})}_{{\rm{Electrodiffusion}}}$](https://content.cld.iop.org/journals/1478-3975/12/5/056007/revision1/pb517942ieqn14.gif) is calculated according to equation (A.31), C represents H+ or
is calculated according to equation (A.31), C represents H+ or  VolM the volume to the medium M (NEURON—n, GLIA—g or EXTRA—o).
VolM the volume to the medium M (NEURON—n, GLIA—g or EXTRA—o).
2.2.2. CO2 hydration
Assuming that [CO2]o is kept constant due to the fact that the extracellular space is an abundant source of CO2 (Chesler 2003, Tong et al 2006) the following reaction was incorporated in the model to investigate its possible contribution for equilibrating the extracellular pH:

Assuming that CO2 hydration is induced by carbonic anhydrase during the carbonic acid (H2CO3) formation (Chesler 2003), and that [CO2]o is constant, the variation rate of [H+]o and [ ]o were calculated by:
]o were calculated by:

where C represents H+ and  νoCA is a proportionality constant determining the reaction speed and
νoCA is a proportionality constant determining the reaction speed and  is the equilibrium reaction constant. The term
is the equilibrium reaction constant. The term ![${{\rm{Kd}}}_{{\rm{CA}}}^{{\rm{o}}}{[{{\rm{CO}}}_{2}]}^{{\rm{o}}}$](https://content.cld.iop.org/journals/1478-3975/12/5/056007/revision1/pb517942ieqn19.gif) was kept constant (=9.56 × 10−4 mM2) to keep the pH at 7.4. νoCA was set in 0.12 mM−1 s−1, this value was calculated based on the equilibrium constant of the reaction
was kept constant (=9.56 × 10−4 mM2) to keep the pH at 7.4. νoCA was set in 0.12 mM−1 s−1, this value was calculated based on the equilibrium constant of the reaction  Kd = 0.27 mM, and the reaction rate of the reaction
Kd = 0.27 mM, and the reaction rate of the reaction  α = 3.32 × 10−2 mM−1 s−1 (Tong et al 2006) :
α = 3.32 × 10−2 mM−1 s−1 (Tong et al 2006) :  .
.
Including equation (7) in the model, equation (3) must then be substituted by :

where

where C is H+ or  .
.
2.2.3. Glial  /Cl− exchanger
/Cl− exchanger
The following mechanisms were considered as potentially responsible for the  regulation (Rufin et al 2014), first assuming it as an exclusive glial mechanism: Na+-coupled
regulation (Rufin et al 2014), first assuming it as an exclusive glial mechanism: Na+-coupled  transporters (NBCT); the
transporters (NBCT); the  /Cl− exchangers (HCE). The first would not be effective in reducing extracellular
/Cl− exchangers (HCE). The first would not be effective in reducing extracellular  since it depends on the [Na+]o. A decrease in [Na+]o during bursts is expected to reduce the action of these transporters. Ionic changes during bursts, on the other hand, may help to account for HCE activity. An increase in [Cl−]i leads to the exchange of intracellular Cl− by the extracellular
since it depends on the [Na+]o. A decrease in [Na+]o during bursts is expected to reduce the action of these transporters. Ionic changes during bursts, on the other hand, may help to account for HCE activity. An increase in [Cl−]i leads to the exchange of intracellular Cl− by the extracellular  which is expected for an extracellular decrease of [
which is expected for an extracellular decrease of [![${{\rm{HCO}}}_{3}^{-}]$](https://content.cld.iop.org/journals/1478-3975/12/5/056007/revision1/pb517942ieqn30.gif) to occur. Therefore, considering that reactions of Cl− and
to occur. Therefore, considering that reactions of Cl− and  with the exchanger enzyme are always at an equilibrium and that the HCE exchange can be reversed, depending on the Cl− and
with the exchanger enzyme are always at an equilibrium and that the HCE exchange can be reversed, depending on the Cl− and  gradients, the following equation was deduced:
gradients, the following equation was deduced:
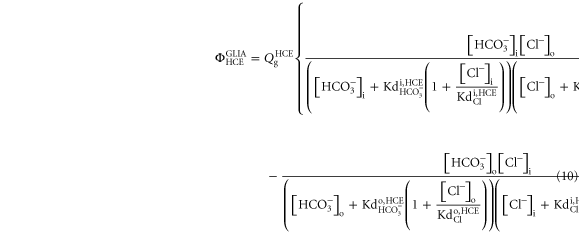
where the constant  allows adjusting the intensity of the exchanger flux,
allows adjusting the intensity of the exchanger flux,  and
and  are the ionic dissociation constant (
are the ionic dissociation constant ( or Cl−) at the intra and extracellular surfaces of the cellular membrane, respectively.
or Cl−) at the intra and extracellular surfaces of the cellular membrane, respectively.
The variation rate of [![${{\rm{HCO}}}_{3}^{-}],$](https://content.cld.iop.org/journals/1478-3975/12/5/056007/revision1/pb517942ieqn37.gif) previously calculated with equation (2), for glial cells and (8) for the extracellular space, was modified to include the glial HCE effect:
previously calculated with equation (2), for glial cells and (8) for the extracellular space, was modified to include the glial HCE effect:
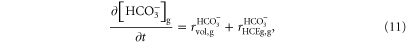

where


 is the membrane area of the glial cell and the term
is the membrane area of the glial cell and the term  of equation (A.36), describing the Cl− flux through glial membrane, was modified:
of equation (A.36), describing the Cl− flux through glial membrane, was modified:

2.2.4. Neuronal  /Cl− exchanger
/Cl− exchanger
To describe HCE exchangers in neurons, the following equation was implemented to the model:
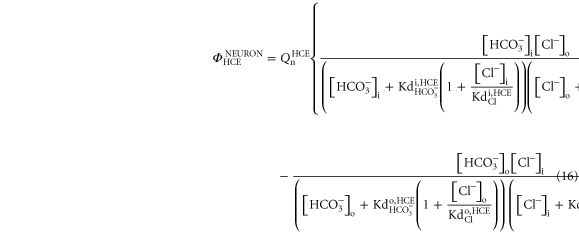
where the constant  similarly to the HCE implement in the glial cells, is used to adjust the HCE flux intensity. The dissociation constants
similarly to the HCE implement in the glial cells, is used to adjust the HCE flux intensity. The dissociation constants  and
and  were set with the same values adopted for the HCE exchangers of glial cells.
were set with the same values adopted for the HCE exchangers of glial cells.
Assuming that neuronal and extracellular ![$[{{{\rm{HCO}}}_{3}}^{-}]$](https://content.cld.iop.org/journals/1478-3975/12/5/056007/revision1/pb517942ieqn44.gif) are respectively calculated according to equations (1) and (12) and estimating that the HCE rate may be appraised by equations (16), (17) was then included in the model and equation (12) was replaced by (18), as follows:
are respectively calculated according to equations (1) and (12) and estimating that the HCE rate may be appraised by equations (16), (17) was then included in the model and equation (12) was replaced by (18), as follows:


where


 is the membrane area of the glial cells and the term
is the membrane area of the glial cells and the term  of equation (A.36), describing the Cl− flux through the neuronal membrane, was altered:
of equation (A.36), describing the Cl− flux through the neuronal membrane, was altered:

In the simulations used,  and
and  were assumed as being equal and adjusted in 6 × 10−3 μ mol cm−2 s−1.
were assumed as being equal and adjusted in 6 × 10−3 μ mol cm−2 s−1.
2.2.5. Neuronal NHE
Assuming the instantaneous equilibrium of the reaction of Na+ and H+ with the exchanger enzyme and the fact that NHE can reverse the direction of the ionic transport (i.e. depending on the Na+ and H+ gradients), the following equation has been derived and implemented in the model:

where  is constant and allows the adjustment of the NHE flux intensity and
is constant and allows the adjustment of the NHE flux intensity and  and
and  are the dissociation constants of the ions (Na+ and H+) in the intra and extracellular surfaces of the neuronal membrane, respectively. The following values were used for these constants:
are the dissociation constants of the ions (Na+ and H+) in the intra and extracellular surfaces of the neuronal membrane, respectively. The following values were used for these constants: 
 65 mM;
65 mM;  = 1.7 × 10−5 mM;
= 1.7 × 10−5 mM;  = 3.8 × 10−5 mM. These values were adjusted to give the best fitting to the experimental data and are in the experimental ranges of the ionic affinities of the corresponding NHE sites described for different tissues (Green et al 1988, Krayer-Pawlowska et al 1991, Orlowski 1993, Orlowski and Grinstein 1997, Szabó et al 2000, Wakabayashi et al 2003).
= 3.8 × 10−5 mM. These values were adjusted to give the best fitting to the experimental data and are in the experimental ranges of the ionic affinities of the corresponding NHE sites described for different tissues (Green et al 1988, Krayer-Pawlowska et al 1991, Orlowski 1993, Orlowski and Grinstein 1997, Szabó et al 2000, Wakabayashi et al 2003).
Since our simulations predicted a neuronal and glial accumulation of  due to the influx of this anion through the HCE and an increase action of the carbonic anhydrase in the intracellular space (Boron 2004, Svichar et al 2009), the following equation was deduced for the estimation of the changes of [H+]i and [
due to the influx of this anion through the HCE and an increase action of the carbonic anhydrase in the intracellular space (Boron 2004, Svichar et al 2009), the following equation was deduced for the estimation of the changes of [H+]i and [ ]i:
]i:

where C represents H+ and  M indicates the neuronal or glial intracellular space,
M indicates the neuronal or glial intracellular space,  is a proportionality constant related to the speed of the reaction in the intracellular space, and
is a proportionality constant related to the speed of the reaction in the intracellular space, and  is the equilibrium constant of the reaction. The term
is the equilibrium constant of the reaction. The term ![${{\rm{Kd}}}_{{\rm{CA}}}^{{\rm{i}}}{[{{\rm{CO}}}_{2}]}^{{\rm{i}}}$](https://content.cld.iop.org/journals/1478-3975/12/5/056007/revision1/pb517942ieqn61.gif) was assumed as being equal to 7.37 × 10−5 mM2 in order to maintain a pH of 7.4 in the resting state in both neurons and in glial cells.
was assumed as being equal to 7.37 × 10−5 mM2 in order to maintain a pH of 7.4 in the resting state in both neurons and in glial cells.
Another mechanism involved in the control of intracellular H+ levels was the H+ pump, present in all regions of the hippocampus (Murata et al 2002, Chesler 2003). This pump is responsible for the efflux of H+, when the pH drops in the intracellular space. As a result, it seems to be indispensable for avoiding the intracellular accumulation of H+. The flux promoted by the H+ pump was determined assuming the instantaneous equilibrium of H+ and ATP with the pump enzyme:

where  is constant and allows to adjust the intensity of the pump flux.
is constant and allows to adjust the intensity of the pump flux.  and
and  are dissociation constants of H+ and ATP in the intracellular face of the membrane.
are dissociation constants of H+ and ATP in the intracellular face of the membrane.  was assumed equal to 5.7 × 10−5 mM, making the pump sensitive to the [H+] intracellular. The H+ pump affinity to ATP was assumed as being equal to the Na/K pump:
was assumed equal to 5.7 × 10−5 mM, making the pump sensitive to the [H+] intracellular. The H+ pump affinity to ATP was assumed as being equal to the Na/K pump:  (Almeida et al 2008).
(Almeida et al 2008).
To include the effect of these mechanisms on the variation rates of [H+] and [![${{{\rm{HCO}}}_{3}}^{-}]$](https://content.cld.iop.org/journals/1478-3975/12/5/056007/revision1/pb517942ieqn67.gif) in neurons, glial cells and extracellular space, it was necessary to add both equations (25) and (26) and to replace equation (9) for H+ by (27) and equations (11) and (12) by (28) and (29), respectively:
in neurons, glial cells and extracellular space, it was necessary to add both equations (25) and (26) and to replace equation (9) for H+ by (27) and equations (11) and (12) by (28) and (29), respectively:




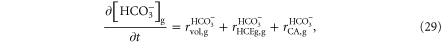
where






where C represents H+ or  With the inclusion of the NHE, the term
With the inclusion of the NHE, the term  of the equation (A.36), that describes the Na+ flux through the neuronal membrane, was modified:
of the equation (A.36), that describes the Na+ flux through the neuronal membrane, was modified:

In the simulations performed, the constants 
 and
and  were assumed equal, respectively, to 5 × 10−10 μ mol cm−2 s−1, 7.0 × 10−3 mM−1 s−1 and 1.2 × 10−10 μ mol cm−2 s−1.
were assumed equal, respectively, to 5 × 10−10 μ mol cm−2 s−1, 7.0 × 10−3 mM−1 s−1 and 1.2 × 10−10 μ mol cm−2 s−1.
2.3. Computational requirements
Computational simulations were performed after describing the equations with finite difference methods and admitting a tolerance error of 10−4. The model was implement in FORTRAN 90. To perform the simulation with a network of 9 × 29 × 19 functional units, the computational code was parallelized and a high performance computer was used (Cluster SGI UV 2000, 80 Cores—Intel Xeon E5-4650v2 10-core, 2.4 GHz, 25MB Cache, RAM 1024GB DDR3 1866 MHz, 80TB HD, SUSE Linux Enterprise Server 11, SGI Performance Suite, Intel Cluster Studio XE).
3. Results
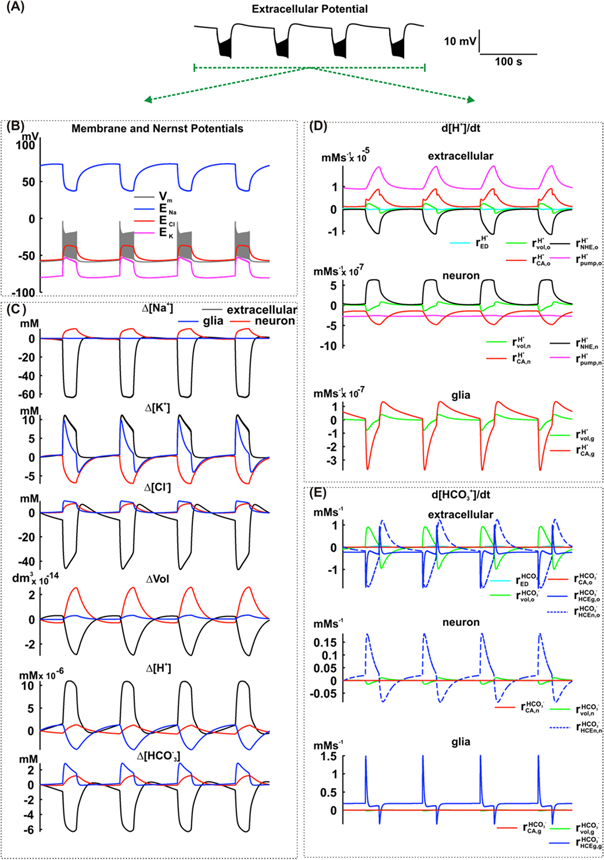
Simulations from Almeida et al (2008) in hippocampal slices showed that when [K+] increases in the bath solution, the diffusion of this ion into the cell body of dentate granule cells was followed by Cl− influx. Confirming previous experimental interpretations, such influx was mediated by an interplay between the functional activity of cotransporters, the Na/K pump and even Cl− channels. The associated intracellular increase of Cl− results in a reduction of the Nernst potential and, as a consequence, neuronal depolarization. Once depolarized and mutually coupled by gap-junctions, the electric field effect and extracellular ionic changes synchronize neuronal network activity and generate nonsynaptic epileptiform bursts. Each burst results in changes similar to those described in both, the original model (Almeida et al 2008) and after experimental induction in hippocampal slices (Xiong and Stringer 2000b). During bursts, dentate granule cells fire action potentials at high frequency (∼40 hz) and the simultaneous field potential exhibits negative dc shifts (figure 2(A)). The intra and extracellular ionic concentrations shown in figure 2(C) include: (i) accumulation of K+ and reduction in Cl− and Na+ in the extracellular space; (ii) reduction in neuronal [K+] and increase in [Cl−] and [Na+]; (iii) small fluctuations in [Na+] and accumulation of Cl− and K+ in glial cells. Simultaneously, increases in neuronal and glial intracellular volumes take place along with corresponding reductions of extracellular volume.
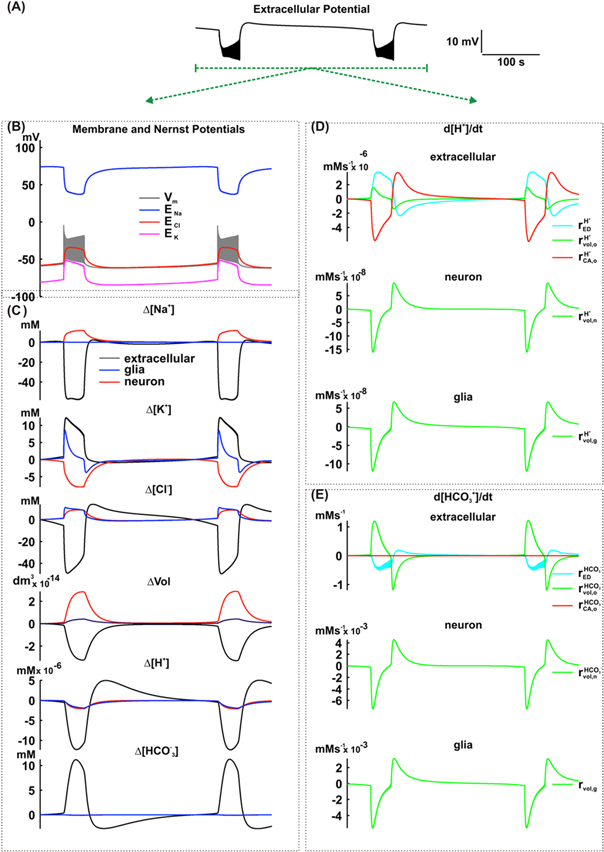
Figure 2. Simulation including in the NEA model H+ and  (A) Extracellular potential. (B) Membrane and Nernst Potentials. (C) Concentration changes (extracellular, neuronal and glial) of Na+, K+, Cl−, H+ and
(A) Extracellular potential. (B) Membrane and Nernst Potentials. (C) Concentration changes (extracellular, neuronal and glial) of Na+, K+, Cl−, H+ and  and also the cellular volumes. (D) Variation rate of the H+ concentration, in the extracellular space (o), neurons (n) and glial cells (g), caused by extracellular electrodiffusion (ED) and volume changes (vol). (E) Variation rate of the
and also the cellular volumes. (D) Variation rate of the H+ concentration, in the extracellular space (o), neurons (n) and glial cells (g), caused by extracellular electrodiffusion (ED) and volume changes (vol). (E) Variation rate of the  concentration, in the extracellular space (o), neurons (n) and glial cells (g), caused by extracellular electrodiffusion (ED) and volume changes (vol).
concentration, in the extracellular space (o), neurons (n) and glial cells (g), caused by extracellular electrodiffusion (ED) and volume changes (vol).
Download figure:
Standard image High-resolution imageThe original NEA model proposed by Almeida et al (2008) and Lopes et al (2013) did not include mechanisms to simulate pH changes. To understand the effects of pH changes on NEAs, the following sequence of mechanisms were incorporated into the model. Simulations were interpreted taking into account experimental findings that allowed us to propose potential mechanisms involved.
3.1. Including H+ and 
As can be observed in figure 2(C), the inclusion of the extracellular electrodiffusion of H+ and  into the model suggest that while [H+]o increases pH decreases. This change is in accordance, at least qualitatively, with the experimental findings from Xiong and Stringer (2000a) (figure 3(A)). However, [H+]i is reduced in simulated neurons, which is not in agreement with the intracellular acidification of the granule cells recorded by Xiong et al (2000) (figure 3(A)). As in the simulated neurons, alkalinization of the cytoplasm has also been observed in simulations of the glial cells (figure 3(B)).
into the model suggest that while [H+]o increases pH decreases. This change is in accordance, at least qualitatively, with the experimental findings from Xiong and Stringer (2000a) (figure 3(A)). However, [H+]i is reduced in simulated neurons, which is not in agreement with the intracellular acidification of the granule cells recorded by Xiong et al (2000) (figure 3(A)). As in the simulated neurons, alkalinization of the cytoplasm has also been observed in simulations of the glial cells (figure 3(B)).
Figure 3. pH changes in neuronal and glial cells as well as in the extracellular space during simulations of NEA. (A) Comparison between simulations of pH changes in extracellular space and in neurons with ictal experimental data extracted from Xiong and Stringer (2000a) and Xiong et al (2000). For each simulation, the inclusion of mechanisms related to changes of [H+] and [![${{\rm{HCO}}}_{3}^{-}]$](https://content.cld.iop.org/journals/1478-3975/12/5/056007/revision1/pb517942ieqn78.gif) are shown as curves related to the simulations of: figure 2—(i); figure 4—(ii); figure 5—(iii); figure 6—(iv); figure 7—(v). (B) For each simulation (i)–(v) are shown as pH changes simultaneous to extracellular potential alterations during the ictal period and the recovery in the interictal. '*' indicates the period of pH change used to compare with the experimental data during the tuning processes. ΔpH was calculated using the pH of the interictal period as the reference.
are shown as curves related to the simulations of: figure 2—(i); figure 4—(ii); figure 5—(iii); figure 6—(iv); figure 7—(v). (B) For each simulation (i)–(v) are shown as pH changes simultaneous to extracellular potential alterations during the ictal period and the recovery in the interictal. '*' indicates the period of pH change used to compare with the experimental data during the tuning processes. ΔpH was calculated using the pH of the interictal period as the reference.
Download figure:
Standard image High-resolution imageThe simulation allowed us to propose mechanism responsible for changes in [H+] and [![${{\rm{HCO}}}_{3}^{-}]$](https://content.cld.iop.org/journals/1478-3975/12/5/056007/revision1/pb517942ieqn79.gif) (figure 2(D)). The extracellular volume reduction contributed to increase of [H+] and [
(figure 2(D)). The extracellular volume reduction contributed to increase of [H+] and [![${{\rm{HCO}}}_{3}^{-}]$](https://content.cld.iop.org/journals/1478-3975/12/5/056007/revision1/pb517942ieqn80.gif) (
( and
and  Increases of extracellular volume, which reduced these ionic concentrations (
Increases of extracellular volume, which reduced these ionic concentrations ( and
and  seem to be responsible for interictal recovery. Extracellular electrodiffusion contributed to reduce the accumulation of these ions during bursts (
seem to be responsible for interictal recovery. Extracellular electrodiffusion contributed to reduce the accumulation of these ions during bursts ( and
and  and to avoid the excessive reduction of ionic concentration in the interictal period (
and to avoid the excessive reduction of ionic concentration in the interictal period ( and
and  The intracellular volume increase reduced [H+] and [
The intracellular volume increase reduced [H+] and [![${{\rm{HCO}}}_{3}^{-}]$](https://content.cld.iop.org/journals/1478-3975/12/5/056007/revision1/pb517942ieqn89.gif) in granule cells and glial cells during the ictal period (
in granule cells and glial cells during the ictal period (

 and
and  Recovery during the interictal period contributed to increase [H+] and [
Recovery during the interictal period contributed to increase [H+] and [![${{{\rm{HCO}}}^{-}}_{3}]$](https://content.cld.iop.org/journals/1478-3975/12/5/056007/revision1/pb517942ieqn94.gif) and to bring the levels of these ions to their corresponding baseline (
and to bring the levels of these ions to their corresponding baseline (

 and
and  > 0).
> 0).
3.2. Including CO2 hydration
The amplitude of extracellular pH changes (>0.15) is often larger in the simulation (figure 3(B)) than in the experimental condition (∼0.1), as recorded by Xiong and Stringer (2000a). Since in the extracellular environment  is shown to accumulate (Chesler 2003), CO2 hydration, a reaction catalyzed by the carbonic anhydrase, may contribute to minimize pH changes. As shown in figure 3(A) (simulation ii), the inclusion of the CO2 hydration reaction catalyzed by the carbonic anhydrase caused extracellular alkalinization during the ictal period. No changes were revealed for either the field potential or the transmembrane and Nernst potentials. Therefore no concentrations of the other ions remained fairly stable (figures 4(A)–(C)). The only change previewed by the simulation was the reduction of [H+] during the ictal period, caused by an increased activity of carbonic anhydrase (
is shown to accumulate (Chesler 2003), CO2 hydration, a reaction catalyzed by the carbonic anhydrase, may contribute to minimize pH changes. As shown in figure 3(A) (simulation ii), the inclusion of the CO2 hydration reaction catalyzed by the carbonic anhydrase caused extracellular alkalinization during the ictal period. No changes were revealed for either the field potential or the transmembrane and Nernst potentials. Therefore no concentrations of the other ions remained fairly stable (figures 4(A)–(C)). The only change previewed by the simulation was the reduction of [H+] during the ictal period, caused by an increased activity of carbonic anhydrase ( figure 4(D)). In the interictal period, enzymatic activity is restored and the reestablished [H+] (
figure 4(D)). In the interictal period, enzymatic activity is restored and the reestablished [H+] ( The extracellular volume reduction contributed to increase [H+]o and [
The extracellular volume reduction contributed to increase [H+]o and [ ]o during the ictal period (
]o during the ictal period ( and
and  figure 4(D)). In the interictal period, when the extracellular volume returns to baseline, this change contributes to [H+]o and [
figure 4(D)). In the interictal period, when the extracellular volume returns to baseline, this change contributes to [H+]o and [ ]o reduction (
]o reduction ( and
and  figures 4(D) and (E)). In the dentate granule cell cytoplasm and also in glial cells, the increased cellular volume causes reductions in both [H+]i and [
figures 4(D) and (E)). In the dentate granule cell cytoplasm and also in glial cells, the increased cellular volume causes reductions in both [H+]i and [ ]i during the ictal period (
]i during the ictal period (

 and
and  In the interictal period, these concentrations are restored to baseline after the recovery of cellular volumes (
In the interictal period, these concentrations are restored to baseline after the recovery of cellular volumes (

 and
and  Extracellular electrodiffusion contributes to ameliorate changes in [H+]o and [
Extracellular electrodiffusion contributes to ameliorate changes in [H+]o and [ ]o: (i) ictal period
]o: (i) ictal period  and
and  (ii) interictal period
(ii) interictal period  and
and  > 0.
> 0.
Figure 4. Simulation including the effect of the carbonic anhydrase in the extracellular space of the NEA model. (A) Extracellular potential. (B) Membrane and Nernst potentials. (C) Concentration changes (extracellular, neuronal and glial) of Na+, K+, Cl−, H+ and  and also the cellular volumes. (D) Variation rate of the H+ concentration in the extracellular space (o), neurons (n) and glial cells (g), caused by extracellular electrodiffusion (ED), volume changes (vol) and carbonic anhydrase (CA). (E) Variation rate of the
and also the cellular volumes. (D) Variation rate of the H+ concentration in the extracellular space (o), neurons (n) and glial cells (g), caused by extracellular electrodiffusion (ED), volume changes (vol) and carbonic anhydrase (CA). (E) Variation rate of the  concentration in the extracellular space (o), neurons (n) and glial cells (g), caused by extracellular electrodiffusion (ED), volume changes (vol) and carbonic anhydrase (CA).
concentration in the extracellular space (o), neurons (n) and glial cells (g), caused by extracellular electrodiffusion (ED), volume changes (vol) and carbonic anhydrase (CA).
Download figure:
Standard image High-resolution image3.3. Including glial  /Cl− exchanger
/Cl− exchanger
Previous simulations show that an increase in carbonic anhydrase activity promotes extracellular alkalinization due to the accumulation of  during bursts. However, experimental findings (figure 3(A)) show an accumulation of H+, as indicated by a pH reduction. Therefore, the previous simulations were lacking mechanisms responsible for removing
during bursts. However, experimental findings (figure 3(A)) show an accumulation of H+, as indicated by a pH reduction. Therefore, the previous simulations were lacking mechanisms responsible for removing  from the extracellular space. Since neuronal acidification and glial alkalinization have been observed (Xiong et al 2000, Chesler 2003), the first hypothesis tested was that of a glial influx of
from the extracellular space. Since neuronal acidification and glial alkalinization have been observed (Xiong et al 2000, Chesler 2003), the first hypothesis tested was that of a glial influx of  which in the model was represented with the implementation of the equation (10). In this equation, the competition of Cl− and
which in the model was represented with the implementation of the equation (10). In this equation, the competition of Cl− and  to bind the exchanger enzyme in each surface of the cellular membrane were also taken into account. To the best of our knowledge, values of these dissociation constants in neuronal tissues have not been estimated. Therefore, values estimated in other tissues were adopted during simulations (Cassel et al 1988, Nord et al 1988, Whitcomb and Ermentrout 2004):
to bind the exchanger enzyme in each surface of the cellular membrane were also taken into account. To the best of our knowledge, values of these dissociation constants in neuronal tissues have not been estimated. Therefore, values estimated in other tissues were adopted during simulations (Cassel et al 1988, Nord et al 1988, Whitcomb and Ermentrout 2004):  =
= 
 =
= 
 was incremented progressively in successive simulations aiming to intensify the influx of
was incremented progressively in successive simulations aiming to intensify the influx of  during the ictal period. However, the increment of
during the ictal period. However, the increment of  was limited by the maximum numerical error accepted in the simulations (1 × 10−4), which limits the adjusted value of
was limited by the maximum numerical error accepted in the simulations (1 × 10−4), which limits the adjusted value of  at 2 × 10−2 μ mol cm−2 s−1 (figure 3(A)—simulation (iii)). For this simulation, the extracellular pH exhibits transient reduction. However, during the ictal period, the pH increases again in the extracellular space and also increases in the neuronal and glial cytoplasm (figure 3(B)—simulation (iii)).
at 2 × 10−2 μ mol cm−2 s−1 (figure 3(A)—simulation (iii)). For this simulation, the extracellular pH exhibits transient reduction. However, during the ictal period, the pH increases again in the extracellular space and also increases in the neuronal and glial cytoplasm (figure 3(B)—simulation (iii)).
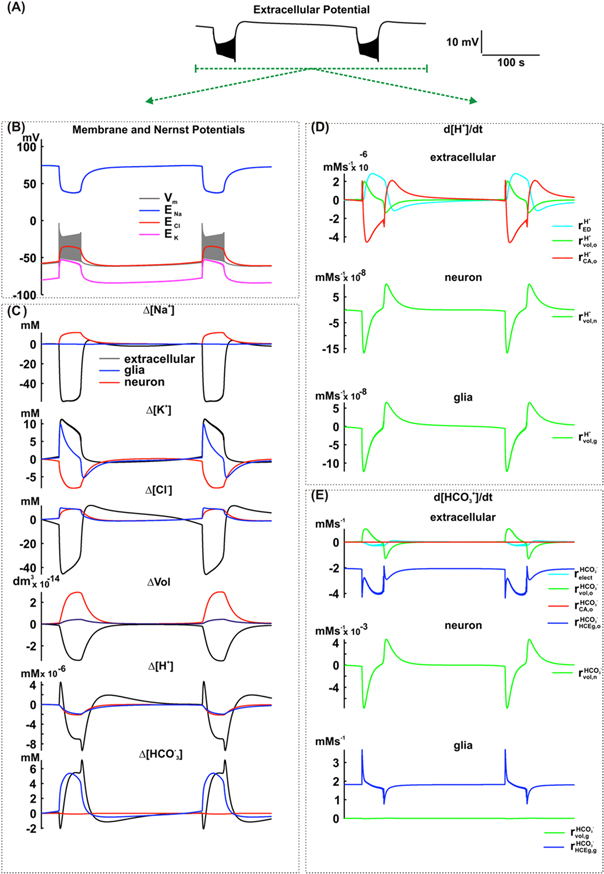
The inclusion of the HCE in glial cells changed the duration of the interictal period (reduction from 227 s to 205 s), but not the ictal period or dc shift (figure 5(A)). In addition, no remarkable changes in cellular volumes, amplitude of the transmembrane or ions Nernst potential were previewed by simulation, as shown in figure 5(B).
Figure 5. Simulation including the effect of the HCE exchanger in glial cells of the NEA model. (A) Extracellular potential. (B) Membrane and Nernst Potentials. (C) Concentration changes (extracellular, neuronal and glial) of Na+, K+, Cl−, H+ and  and also the cellular volumes. (D) Variation rate of the H+ concentration in the extracellular space (o), neurons (n) and glial cells (g), caused by extracellular electrodiffusion (ED), volume changes (vol) and carbonic anhydrase (CA). (E) Variation rate of the
and also the cellular volumes. (D) Variation rate of the H+ concentration in the extracellular space (o), neurons (n) and glial cells (g), caused by extracellular electrodiffusion (ED), volume changes (vol) and carbonic anhydrase (CA). (E) Variation rate of the  concentration, in the extracellular space (o), neurons (n) and glial cells (g), caused by extracellular electrodiffusion (ED), volume changes (vol), carbonic anhydrase (CA) and HCE exchanger in glial cells (HCEg).
concentration, in the extracellular space (o), neurons (n) and glial cells (g), caused by extracellular electrodiffusion (ED), volume changes (vol), carbonic anhydrase (CA) and HCE exchanger in glial cells (HCEg).
Download figure:
Standard image High-resolution imageIn respect to changes in [H+] or [![${{\rm{HCO}}}_{3}^{-}],$](https://content.cld.iop.org/journals/1478-3975/12/5/056007/revision1/pb517942ieqn139.gif) two main aspects must the highlighted: (i) during the ictal period, [H+]o had a transient positive change followed by a progressive reduction. Simultaneously, after an initial drop, [
two main aspects must the highlighted: (i) during the ictal period, [H+]o had a transient positive change followed by a progressive reduction. Simultaneously, after an initial drop, [ ]o tends to increase. In neurons, [H+] and [
]o tends to increase. In neurons, [H+] and [![${{\rm{HCO}}}_{3}^{-}]$](https://content.cld.iop.org/journals/1478-3975/12/5/056007/revision1/pb517942ieqn141.gif) decrease. In glia, while a decrease is observed in [H+], [
decrease. In glia, while a decrease is observed in [H+], [![${{\rm{HCO}}}_{3}^{-}]$](https://content.cld.iop.org/journals/1478-3975/12/5/056007/revision1/pb517942ieqn142.gif) is increased; (iii) in the interictal period, [H+]o and [
is increased; (iii) in the interictal period, [H+]o and [ ]o return to baseline. However, [H+]o had an initial overshoot and [
]o return to baseline. However, [H+]o had an initial overshoot and [ ]o an undershoot. While [H+]i and [
]o an undershoot. While [H+]i and [ ]i went back to their respective baseline, an initial undershoot was observed in glial cells.
]i went back to their respective baseline, an initial undershoot was observed in glial cells.
While investigating the mechanisms responsible for changes in [H+] and [![${{\rm{HCO}}}_{3}^{-}],$](https://content.cld.iop.org/journals/1478-3975/12/5/056007/revision1/pb517942ieqn146.gif) as shown in figures 5(D) and (E), relevant observations could be extracted from simulations concerning the ictal and interictal periods. During the ictal period, similar to the simulations described in the item 3.2, volume changes caused both H+ and
as shown in figures 5(D) and (E), relevant observations could be extracted from simulations concerning the ictal and interictal periods. During the ictal period, similar to the simulations described in the item 3.2, volume changes caused both H+ and  accumulation in the extracellular space (
accumulation in the extracellular space ( and
and  and a cytoplasmic reduction in neurons and glial cells (
and a cytoplasmic reduction in neurons and glial cells (

 and
and  The extracellular electrodiffusion contributed for the [H+]o increase (
The extracellular electrodiffusion contributed for the [H+]o increase ( and [
and [ ]o decrease (
]o decrease ( The carbonic anhydrase caused an initial [H+]o increase (
The carbonic anhydrase caused an initial [H+]o increase ( at the beginning of the ictal period) and later promoted its reduction (
at the beginning of the ictal period) and later promoted its reduction ( in almost all of the ictal period). Carbonic anhydrase had no significant effect on the extracellular concentration of
in almost all of the ictal period). Carbonic anhydrase had no significant effect on the extracellular concentration of  (
( when compared with the changes caused by the other mechanisms. However, the HCE exchanger was responsible for the
when compared with the changes caused by the other mechanisms. However, the HCE exchanger was responsible for the  influx in glial cells (
influx in glial cells ( and
and  < 0).
< 0).
During the interictal period, volume changes reduced [H+]o and [ ]o (
]o ( and
and  and contributed to the cytoplasmic increase of ionic concentrations in neurons and glial cells (
and contributed to the cytoplasmic increase of ionic concentrations in neurons and glial cells (

 and
and  The extracellular electrodiffusion favored the [H+]o increase (
The extracellular electrodiffusion favored the [H+]o increase ( Even during the interictal period, the HCE exchanger was still responsible for the
Even during the interictal period, the HCE exchanger was still responsible for the  influx into glial cells (
influx into glial cells ( and
and  however, with less intensity.
however, with less intensity.
Due to the action of the carbonic anhydrase, [ ]o seems to be necessary for [H+]o increase (extracellular pH reduction). As shown in figure 5(C),
]o seems to be necessary for [H+]o increase (extracellular pH reduction). As shown in figure 5(C),  influx into glial cells was not enough to reduce [
influx into glial cells was not enough to reduce [ ]o during the whole ictal period. Despite the initial increase due to glial [Cl−]i, HCE activity was later decreased following a [Cl−]o reduction. This [Cl−]o reduction was promoted by the Cl− influx into neurons and glial cells. Therefore, in neurons, simulation also showed Cl− accumulation (figure 5(C)).
]o during the whole ictal period. Despite the initial increase due to glial [Cl−]i, HCE activity was later decreased following a [Cl−]o reduction. This [Cl−]o reduction was promoted by the Cl− influx into neurons and glial cells. Therefore, in neurons, simulation also showed Cl− accumulation (figure 5(C)).
3.4. Including neuronal  /Cl− exchanger
/Cl− exchanger
Considering that the HCE exchangers are also expressed in neurons (Rufin et al 2014), the neuronal  influx can also occur and would also contribute to the reduction of
influx can also occur and would also contribute to the reduction of  concentration in the extracellular space as well as the regulation of pH. The inclusion of this exchanger in the neurons allows the reproductions of the pH excursion similar to those recorded in the extracellular space (figure 3(A)—simulation (iv)). In addition, it did not affect the typical excursion of the field potential, the transmembrane potential or Nernst potentials when compared with previous simulations (figures 6(A) and (B)). However, the simulation shows a decrease in dc shift as well as the duration of ictal and interictal periods. In contrast, no remarkable changes were observed in the intra and extracellular concentrations of Na+ and K+, as well as in intra and extracellular volumes (figure 6(C)). The inclusion of HCE exchangers affected the extracellular Cl−, resulting in the slow accumulation of this ion during the ictal period (due to the exchange of extracellular
concentration in the extracellular space as well as the regulation of pH. The inclusion of this exchanger in the neurons allows the reproductions of the pH excursion similar to those recorded in the extracellular space (figure 3(A)—simulation (iv)). In addition, it did not affect the typical excursion of the field potential, the transmembrane potential or Nernst potentials when compared with previous simulations (figures 6(A) and (B)). However, the simulation shows a decrease in dc shift as well as the duration of ictal and interictal periods. In contrast, no remarkable changes were observed in the intra and extracellular concentrations of Na+ and K+, as well as in intra and extracellular volumes (figure 6(C)). The inclusion of HCE exchangers affected the extracellular Cl−, resulting in the slow accumulation of this ion during the ictal period (due to the exchange of extracellular  by intracellular Cl−; figure 6(E) -
by intracellular Cl−; figure 6(E) - In the extracellular space, an increase in [H+] (figure 6(C)) has also contributed to a pH decrease (figure 3(B)). In the interictal period, [H+] is re-established to baseline levels. In the neuronal and glial cytoplasm, [H+]i is reduced during the ictal period and only returns to baseline values during the interictal period. Reductions in [
In the extracellular space, an increase in [H+] (figure 6(C)) has also contributed to a pH decrease (figure 3(B)). In the interictal period, [H+] is re-established to baseline levels. In the neuronal and glial cytoplasm, [H+]i is reduced during the ictal period and only returns to baseline values during the interictal period. Reductions in [ ]o during the ictal period are also observed with this ion returning to baseline levels during the interictal phase following an initial overshoot. In neurons and glial cells, [
]o during the ictal period are also observed with this ion returning to baseline levels during the interictal phase following an initial overshoot. In neurons and glial cells, [ ]i increases during the ictal period and recovers during the interictal phase.
]i increases during the ictal period and recovers during the interictal phase.
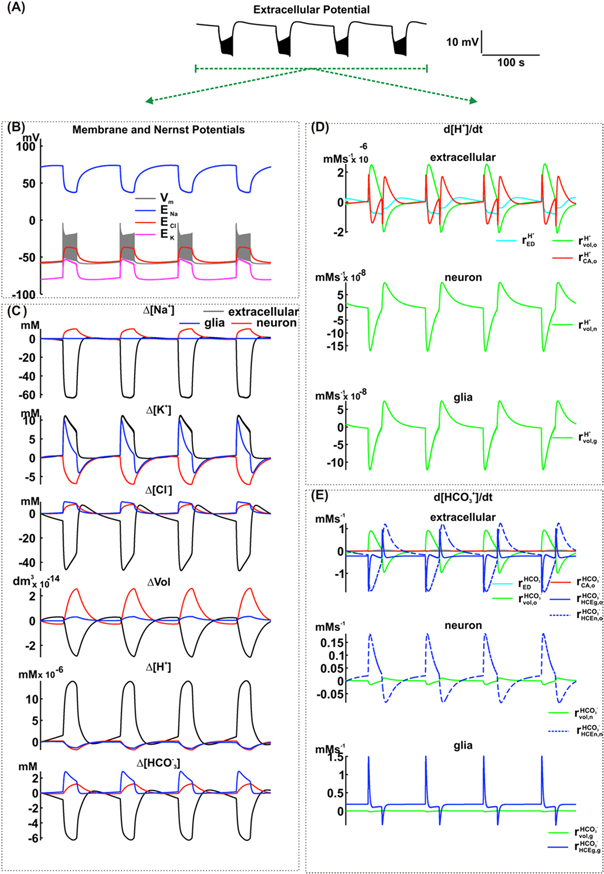
Figure 6. Simulation including the effect of the HCE exchanger in neurons of the NEA model. (A) Potencial extracellular. (B) Membrane and Nernst Potentials. (C) Concentration changes (extracellular, neuronal and glial) of Na+, K+, Cl−, H+ and  and also the cellular volumes. (D) Variation rate of the H+ concentration, in the extracellular space (o), neurons (n) and glial cells (g), caused by extracellular electrodiffusion (ED), volume changes (vol) and carbonic anhydrase (CA). (E) Variation rate of the
and also the cellular volumes. (D) Variation rate of the H+ concentration, in the extracellular space (o), neurons (n) and glial cells (g), caused by extracellular electrodiffusion (ED), volume changes (vol) and carbonic anhydrase (CA). (E) Variation rate of the  concentration, in the extracellular space (o), neurons (n) and glial cells (g), caused by extracellular electrodiffusion (ED), volume changes (vol), carbonic anhydrase (CA), HCE exchanger in glial cells (HCEg) and HCE exchanger in neurons (HCEn).
concentration, in the extracellular space (o), neurons (n) and glial cells (g), caused by extracellular electrodiffusion (ED), volume changes (vol), carbonic anhydrase (CA), HCE exchanger in glial cells (HCEg) and HCE exchanger in neurons (HCEn).
Download figure:
Standard image High-resolution imagePotential mechanisms responsible for [H+] and ![$[{{\rm{HCO}}}_{3}^{-}]$](https://content.cld.iop.org/journals/1478-3975/12/5/056007/revision1/pb517942ieqn187.gif) changes during ictal and interictal periods are shown in figures 6(D) and (E). During the ictal phase, carbonic anhydrase contributed to the initial transient increase of the [H+]o (
changes during ictal and interictal periods are shown in figures 6(D) and (E). During the ictal phase, carbonic anhydrase contributed to the initial transient increase of the [H+]o ( and its subsequent reduction (
and its subsequent reduction ( in the extracellular space. As in previous simulations, the extracellular volume reduction favored H+ accumulation (
in the extracellular space. As in previous simulations, the extracellular volume reduction favored H+ accumulation ( an effect that is countered by electrodiffusion (
an effect that is countered by electrodiffusion ( In the neuronal and glial intracellular spaces, the volume increase reduced [H+]. Analyzing [
In the neuronal and glial intracellular spaces, the volume increase reduced [H+]. Analyzing [ ]o, the simulation does not show expressive activity of the carbonic anhydrase, when compared with the changes caused by the other mechanisms (
]o, the simulation does not show expressive activity of the carbonic anhydrase, when compared with the changes caused by the other mechanisms ( ≪
≪ 

 and
and  The volume changes made the [
The volume changes made the [ ]o reduction less intense (
]o reduction less intense ( and also reduced anionic accumulation in neurons and glial cells (
and also reduced anionic accumulation in neurons and glial cells ( and
and  The neuronal and glial HCE exchangers contributed to the [HCO3]o reduction (
The neuronal and glial HCE exchangers contributed to the [HCO3]o reduction ( and
and  and the anionic accumulation in neurons (
and the anionic accumulation in neurons ( and glial cells (
and glial cells ( > 0).
> 0).
During the interictal period, simulation shows the carbonic anhydrase promoting an initial transient reduction of [H+]o ( followed by increase (
followed by increase ( The extracellular volume recovery contributed to reduce [H+]o (
The extracellular volume recovery contributed to reduce [H+]o ( The extracellular electrodiffusion was initially responsible for the [H+]o reduction (
The extracellular electrodiffusion was initially responsible for the [H+]o reduction ( Subsequently, the electrodiffusion countered the undershoot of [H+]o (
Subsequently, the electrodiffusion countered the undershoot of [H+]o ( In neuronal and glial intracellular spaces, the reinstated volume contributed to the return of [H+]i to baseline levels (
In neuronal and glial intracellular spaces, the reinstated volume contributed to the return of [H+]i to baseline levels (
 On the other hand, the volume change slowed [
On the other hand, the volume change slowed [![${{\rm{HCO}}}_{3}^{-}]$](https://content.cld.iop.org/journals/1478-3975/12/5/056007/revision1/pb517942ieqn213.gif) recovery in the extracellular space, as well as in neurons and glial cells (
recovery in the extracellular space, as well as in neurons and glial cells (
 and
and  [
[![${{\rm{HCO}}}_{3}^{-}]$](https://content.cld.iop.org/journals/1478-3975/12/5/056007/revision1/pb517942ieqn217.gif) recovery was mediated by neuronal and glial HCE exchangers (
recovery was mediated by neuronal and glial HCE exchangers (

 and
and  < 0).
< 0).
3.5. Including neuronal NHE
Comparing the simulated pH changes with the experimental data from Xiong and Stringer (2000a), it can be seen that changes in the extracellular space are more intense in the simulation (ΔpH ∼ 0,15, figure 3(B), simulation (iv)) than in the recordings (ΔpH ∼ 0,1). Moreover, as shown in figure 3(B) (simulation (iv)), the simulation shows neuronal intracellular alkalinization, whereas the experimental recording reveal acidification (figure 3(A)). Therefore, it is appropriate to suspect that mechanisms able to promote H+ influx in neurons are required. According to Chesler (2003), the Na+/H+ exchanger (NHE) is vastly expressed in the brain. This mechanism is normally described as an acid extruder, due to the elevated level of extracellular Na+ that contributes to the outflow of H+ in normal conditions (Chesler 2003, Ruffin et al 2014). However, under specific circumstances that effectively reduce Na+ this exchanger can cause H+ influx (Cha et al 2009). As shown in figure 3(A) (simulation (v)), the inclusion of NHE in the model allow to simulate the dynamic of the pH excursion of the intra and extracellular spaces, as observed experimentally (Xiong and Stringer 2000). The amplitude of changes was also reproduced in the same range. In glial cells, the model shows alkalinization associated with the intracellular  accumulation. Our simulation suggested that the inclusion of the NHE and H+ pump and of the effects of carbonic anhydrase on the extracellular space in the model did not alter the dynamic of the field potential (figure 7(A)), the transmembrane potential, the Nernst potentials (figure 7(B)), ionic concentrations in intra and extracellular spaces and cellular volume changes (figure 7(C)). Remarkable changes were related to the [H+] in the extracellular space and also in the neuronal and glial intracellular spaces. These changes are not enough to alter the concentration of other ions, since the mechanism responsible for [H+] changes are less intense than the activities of the mechanisms that affect their concentrations. Comparing the effects of mechanisms that changed [H+] and [
accumulation. Our simulation suggested that the inclusion of the NHE and H+ pump and of the effects of carbonic anhydrase on the extracellular space in the model did not alter the dynamic of the field potential (figure 7(A)), the transmembrane potential, the Nernst potentials (figure 7(B)), ionic concentrations in intra and extracellular spaces and cellular volume changes (figure 7(C)). Remarkable changes were related to the [H+] in the extracellular space and also in the neuronal and glial intracellular spaces. These changes are not enough to alter the concentration of other ions, since the mechanism responsible for [H+] changes are less intense than the activities of the mechanisms that affect their concentrations. Comparing the effects of mechanisms that changed [H+] and [![${{\rm{HCO}}}_{3}^{-}]$](https://content.cld.iop.org/journals/1478-3975/12/5/056007/revision1/pb517942ieqn223.gif) with the previous simulations (figures 6(D) and (E)), there were no significant changes in [
with the previous simulations (figures 6(D) and (E)), there were no significant changes in [![${{\rm{HCO}}}_{3}^{-}].$](https://content.cld.iop.org/journals/1478-3975/12/5/056007/revision1/pb517942ieqn224.gif) The changes were only relevant for [H+] (figure 7(E)).
The changes were only relevant for [H+] (figure 7(E)).
Figure 7. Simulation including the effect of the NHE exchanger and the H+ pump in neurons of the NEA model. (A) Extracellular potential. (B) Membrane and Nernst Potentials. (C) Concentration changes (extracellular, neuronal and glial) of Na+, K+, Cl−, H+ and  and also the cellular volumes. (D) Variation rate of H+ concentration in the extracellular space (o), neurons (n) and glial cells (g), caused by extracellular electrodiffusion (ED), volume changes (vol), carbonic anhydrase (CA), NHE exchanger in neurons and H+ pump in neurons. (E) Variation rate of
and also the cellular volumes. (D) Variation rate of H+ concentration in the extracellular space (o), neurons (n) and glial cells (g), caused by extracellular electrodiffusion (ED), volume changes (vol), carbonic anhydrase (CA), NHE exchanger in neurons and H+ pump in neurons. (E) Variation rate of  concentration in the extracellular space (o), neurons (n) and glial cells (g), caused by extracellular electrodiffusion (ED), volume changes (vol), carbonic anhydrase (CA), HCE exchanger in glial cells (HCEg), HCE exchanger in neurons (HCEn).
concentration in the extracellular space (o), neurons (n) and glial cells (g), caused by extracellular electrodiffusion (ED), volume changes (vol), carbonic anhydrase (CA), HCE exchanger in glial cells (HCEg), HCE exchanger in neurons (HCEn).
Download figure:
Standard image High-resolution imageThe simulation suggests that, in the ictal period, the extracellular volume change, the activity of the carbonic anhydrase, and the H+ pump are influenced by H+ accumulation (

 The NHE, which transports H+ to the neuronal intracellular space (
The NHE, which transports H+ to the neuronal intracellular space ( is counteracted, which reduces H+ accumulation. Compared to these mechanisms, electrodiffusion did not have a significant effect (
is counteracted, which reduces H+ accumulation. Compared to these mechanisms, electrodiffusion did not have a significant effect ( In neurons, the NHE causes [H+] to increase (
In neurons, the NHE causes [H+] to increase ( which is restricted by the effects of the neuronal volume increase and the activities of the carbonic anhydrase and H+ pump (
which is restricted by the effects of the neuronal volume increase and the activities of the carbonic anhydrase and H+ pump (

 In glial cells, the volume increase and the carbonic anhydrase activity reduce the [H+].
In glial cells, the volume increase and the carbonic anhydrase activity reduce the [H+].
During the interictal period, the simulation proposes that the carbonic anhydrase and the H+ pump continue contributing for the [H+]o increase with a lesser intensity (
 The re-establishment of the extracellular volume and of NHE activity restricted [H+] increase in the extracellular space. In neurons, the NHE and the cell volume reduction delayed the [H+] decrease (
The re-establishment of the extracellular volume and of NHE activity restricted [H+] increase in the extracellular space. In neurons, the NHE and the cell volume reduction delayed the [H+] decrease (
 The H+ pump and the carbonic anhydrase are responsible for the neuronal [H+] recovering (
The H+ pump and the carbonic anhydrase are responsible for the neuronal [H+] recovering (
 In glial cells, the [H+] recovery is due to the carbonic anhydrase activity and the cell volume reduction.
In glial cells, the [H+] recovery is due to the carbonic anhydrase activity and the cell volume reduction.
4. Discussion
In the present work we have used computational simulations to investigate mechanisms potentially responsible for changes in pH during NEA. We propose a group of coupled mechanisms that, when incorporated to the NEA model (Almeida et al 2008), reproduces experimental findings (Xiong et al 2000, Xiong and Stringer (2000a), (2000b)). Our simulations show that the following mechanisms may play a crucial role in promoting pH changes: extracellular electrodiffusion, cellular volume changes, CO2 hydration (catalyzed by the carbonic anhydrase), the NHE exchanger, the HCE exchanger and the H+ pump.
The main effect of extracellular electrodiffusion is to reduce the amplitude of the variations in ionic concentrations of H+ and  In the interictal period, electrodiffusion contributes to reestablish ionic concentrations of these ions and, therefore, for the recovery of a normal pH. This effect, however, is less powerful than that caused by mechanisms that directly change [H+]o and [
In the interictal period, electrodiffusion contributes to reestablish ionic concentrations of these ions and, therefore, for the recovery of a normal pH. This effect, however, is less powerful than that caused by mechanisms that directly change [H+]o and [ ]o (figure 7).
]o (figure 7).
The ictal period of NEA is characterized by an increase in the intracellular volume of neurons and glia, and a concomitant reduction in the extracellular volume (figures 2, 4–7). Such an increase in neuronal volume reduces both H+ accumulation and intracellular acidification. Similarly, a volumetric increase of glial cells leads to intracellular alkalinization. Extracellular volume reduction may cause either a direct increase (figure 2) or a mild reduction (figures 4–7) in [H+] and [![${{\rm{HCO}}}_{3}^{-}]$](https://content.cld.iop.org/journals/1478-3975/12/5/056007/revision1/pb517942ieqn244.gif) (figures 5–7). Taking into account the coordinated effect of carbonic anhydrase, an increase in [
(figures 5–7). Taking into account the coordinated effect of carbonic anhydrase, an increase in [ ]o promoted by an extracellular volume reduction favors the production of CO2 and H2O. This ultimately results in [H+]o reduction (pH increase) (figure 4). When the extracellular volume reduction produces only a mild decrease in
]o promoted by an extracellular volume reduction favors the production of CO2 and H2O. This ultimately results in [H+]o reduction (pH increase) (figure 4). When the extracellular volume reduction produces only a mild decrease in ![${[{{\rm{HCO}}}_{3}^{-}]}_{{\rm{o}}},$](https://content.cld.iop.org/journals/1478-3975/12/5/056007/revision1/pb517942ieqn246.gif) it results in less extracellular acidification (figures 5–7). Therefore, our simulations suggest that, when investigating mechanisms of H+ and
it results in less extracellular acidification (figures 5–7). Therefore, our simulations suggest that, when investigating mechanisms of H+ and  transport on pH during intense neuronal activation (e.g. during NEA), the effect of extracellular volume changes must be taken into account.
transport on pH during intense neuronal activation (e.g. during NEA), the effect of extracellular volume changes must be taken into account.
At the extracellular space, the conversion rate between H+– and CO2–H2O is increased by the catalytic action of carbonic anhydrase (Lindskog and Coleman 1973, Maren 1967). Using parameters for the action of the carbonic anhydrase estimated experimentally (Tong et al 2006), the current simulations suggest that this enzyme causes an extracellular increase of [H+] associated with a reduction in [
and CO2–H2O is increased by the catalytic action of carbonic anhydrase (Lindskog and Coleman 1973, Maren 1967). Using parameters for the action of the carbonic anhydrase estimated experimentally (Tong et al 2006), the current simulations suggest that this enzyme causes an extracellular increase of [H+] associated with a reduction in [ ]o due to neuronal and glial
]o due to neuronal and glial  influx through the HCE (figures 5–7). This may contribute to the reduction in extracellular pH observed in experimental preparations (figure 3). According to Xiong and Stringer 2000b and Chesler 2003, a possible mechanism for the acidification of extracellular space accompanying the epileptiform activities is resultant from increased glial metabolism. However, the present simulations suggest an alternative explanation. HCE captures
influx through the HCE (figures 5–7). This may contribute to the reduction in extracellular pH observed in experimental preparations (figure 3). According to Xiong and Stringer 2000b and Chesler 2003, a possible mechanism for the acidification of extracellular space accompanying the epileptiform activities is resultant from increased glial metabolism. However, the present simulations suggest an alternative explanation. HCE captures  into neurons and glial cells, resulting in [
into neurons and glial cells, resulting in [ ]o reduction. Such a reduction would then favor the carbonate hydration catalyzed by carbonic anhydrase, causing an extracellular pH reduction.
]o reduction. Such a reduction would then favor the carbonate hydration catalyzed by carbonic anhydrase, causing an extracellular pH reduction.
One could also argue that Na+-dependent transporters NBCT, additionally to HCE, may also play a role on  influx, as reported during physiological brain conditions (Rufin et al 2014). However, as already mentioned in the Results section, the decrease in [Na+]o would go against this hypothesis. In addition, the neuronal Na+ accumulation that occurs following action potentials via voltage dependent channels would diminish the possible
influx, as reported during physiological brain conditions (Rufin et al 2014). However, as already mentioned in the Results section, the decrease in [Na+]o would go against this hypothesis. In addition, the neuronal Na+ accumulation that occurs following action potentials via voltage dependent channels would diminish the possible  influx throughout these transporters. In glial cells, Na+ influx has not been observed during the ictal period (Xiong and Stringer 2000). Therefore, the current simulations suggest that HCE is the main mechanism responsible for the
influx throughout these transporters. In glial cells, Na+ influx has not been observed during the ictal period (Xiong and Stringer 2000). Therefore, the current simulations suggest that HCE is the main mechanism responsible for the  influx in glial cells and neurons. During NEA, the intracellular Cl− accumulation and its extracellular reduction favored the action of the HCE.
influx in glial cells and neurons. During NEA, the intracellular Cl− accumulation and its extracellular reduction favored the action of the HCE.
According to several reports, prolonged neuronal activity such as the one observed in NEA may be accompanied by intracellular decrease in pH due to different mechanisms (Meech and Thomas 1977, Thomas and Meech 1982, Siesjö et al 1985, Kaila and Voipio, 1987, Chen and Chesler 1992, Wang et al 1994): (i) metabolic production of CO2 and lactate; (ii) acid influx via ionic channels and Ca2+ -dependent processes. The simulations performed here suggest a simple explanation. Prolonged neuronal activity causes intracellular Na+ accumulation. This leads to the activation of NHE in the reverse direction, which causes H+ influx and intracellular pH reductions. Moreover, our simulations show that the H+ pump and carbonic anhydrase reduce neuronal H+, hence contributing to pH control during epileptiform events.
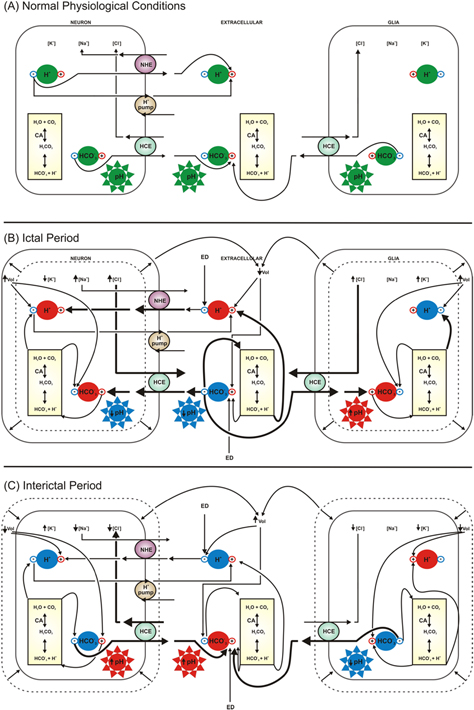
In summary, the simulations carried out in the present work have allowed us to propose the following (figure 8(A)): under normal physiological conditions, the intracellular (neurons and glial cells) and extracellular concentrations of H+ are governed by NHE, which causes an outflow of H+ and an inflow of Na+. The H+ pump contributes to the extrusion of H+ while the HCE captures Cl− and extrudes  The cellular volume, extracellular electrodiffusion and carbonic anhydrase have no significant effect on the pH, which is maintained at a normal level.
The cellular volume, extracellular electrodiffusion and carbonic anhydrase have no significant effect on the pH, which is maintained at a normal level.
Figure 8. Schemes of the proposed explanation for pH changes during normal physiological conditions, ictal and interictal periods in neurons, glial cells and extracellular space. Arrows indicate the effect of each mechanism considered in the model, that is responsible for increasing (+) or decreasing (−)H+ and  concentrations. Colors in in the symbols of H+,
concentrations. Colors in in the symbols of H+,  and pH indicate a normal physiological condition (green), increased (red) or decreased (blue) levels. Stronger effects are represented by thicker arrows. In normal physiological conditions (A), [H+]o and [H+]i are governed by NHE, causing H+ outflow and Na+ inflow. The H+ pump is involved in H+ extrusion while HCE captures Cl− and extrudes
and pH indicate a normal physiological condition (green), increased (red) or decreased (blue) levels. Stronger effects are represented by thicker arrows. In normal physiological conditions (A), [H+]o and [H+]i are governed by NHE, causing H+ outflow and Na+ inflow. The H+ pump is involved in H+ extrusion while HCE captures Cl− and extrudes  The liquid effect is then what maintains the pH in a normal range. In the ictal period of the NEA (B), exchange of Na+ and H+ by the NHE is reversed by the intracellular accumulation of Na+, due to neuronal depolarization. The HCE is reversed by [Cl−] gradient, therefore promoting Cl− extrusion and an inflow of
The liquid effect is then what maintains the pH in a normal range. In the ictal period of the NEA (B), exchange of Na+ and H+ by the NHE is reversed by the intracellular accumulation of Na+, due to neuronal depolarization. The HCE is reversed by [Cl−] gradient, therefore promoting Cl− extrusion and an inflow of 
 from the extracellular space. This scenario increases carbonic anhydrase activity, H+ pumping and intracellular volume, attenuating H+ accumulation. The final effect is a pH decrease. In glial cells, changes in the [Cl−] gradient reverse HCE exchange. A [
from the extracellular space. This scenario increases carbonic anhydrase activity, H+ pumping and intracellular volume, attenuating H+ accumulation. The final effect is a pH decrease. In glial cells, changes in the [Cl−] gradient reverse HCE exchange. A [ ]I increase activates the carbonic anhydrase, catalyzing the carbonate hydration. The final effect is the pH increase in the glial cells. In the extracellular space, [
]I increase activates the carbonic anhydrase, catalyzing the carbonate hydration. The final effect is the pH increase in the glial cells. In the extracellular space, [![${{\rm{HCO}}}_{3}^{-}]$](https://content.cld.iop.org/journals/1478-3975/12/5/056007/revision1/pb517942ieqn263.gif) decreases concomitantly. This activates the carbonic anhydrase, catalyzing the conversion of carbonate in
decreases concomitantly. This activates the carbonic anhydrase, catalyzing the conversion of carbonate in  and H+ and leading to the extracellular decrease of pH. During the interictal period of the NEA (C), the recovery of the ionic levels reverses the HCE and results in
and H+ and leading to the extracellular decrease of pH. During the interictal period of the NEA (C), the recovery of the ionic levels reverses the HCE and results in  outflow. The recovery of [
outflow. The recovery of [ ]o to the baseline reduces the carbonic anhydrase activity and contributes to the extracellular H+ recovery. The reduction in the neuronal Na+ gradient decreases NHE activity and increases the neuronal pH. Although the reduction in NHE activity promotes an extracellular accumulation of H+, the reduction of carbonic anhydrase activation is more intense and results in an extracellular pH increase. Extracellular volume changes and the electrodiffusion effects are countered and do not modulate pH changes. In glial cells, the return of ionic concentrations to baseline levels reverses the HCE and leads to intracellular reduction of [
]o to the baseline reduces the carbonic anhydrase activity and contributes to the extracellular H+ recovery. The reduction in the neuronal Na+ gradient decreases NHE activity and increases the neuronal pH. Although the reduction in NHE activity promotes an extracellular accumulation of H+, the reduction of carbonic anhydrase activation is more intense and results in an extracellular pH increase. Extracellular volume changes and the electrodiffusion effects are countered and do not modulate pH changes. In glial cells, the return of ionic concentrations to baseline levels reverses the HCE and leads to intracellular reduction of [![${{\rm{HCO}}}_{3}^{-}].$](https://content.cld.iop.org/journals/1478-3975/12/5/056007/revision1/pb517942ieqn267.gif) This subsequently induces a reversion in the reaction catalyzed by carbonic anhydrase. Under these circumstances, intracellular [H+] increases, an effect that is enhanced by the glial volume reduction.
This subsequently induces a reversion in the reaction catalyzed by carbonic anhydrase. Under these circumstances, intracellular [H+] increases, an effect that is enhanced by the glial volume reduction.
Download figure:
Standard image High-resolution imageDuring the ictal period, our simulations suggest that the induction of NEA with high [K+] induces a neuronal influx of Na+ and Cl− (figure 8(B)). The former occurs though voltage-dependent channels whereas the latter depends on KCC cotransporters. The gradient established for Na+ reverses the exchange of Na+ and H+ by the NHE, resulting in intracellular acidification. The gradient for Cl− is also able to reverse HCE, leading to an extrusion of Cl− and an inflow of  from the extracellular space. Under these circumstances, carbonic anhydrase, H+ pump and an intracellular volumetric increase become important mechanisms to attenuate a raise in intracellular H+ and a drop in pH. In contrast, changes in the glial Cl− gradient promote a reverse HCE exchange. This would in fact be one of the main mechanisms responsible for extruding Cl− and capturing
from the extracellular space. Under these circumstances, carbonic anhydrase, H+ pump and an intracellular volumetric increase become important mechanisms to attenuate a raise in intracellular H+ and a drop in pH. In contrast, changes in the glial Cl− gradient promote a reverse HCE exchange. This would in fact be one of the main mechanisms responsible for extruding Cl− and capturing  Under these circumstances, an internal increase in
Under these circumstances, an internal increase in  induces carbonic anhydrase activation, which catalyzes carbonate hydration. The end result of this process is a lower [H+]i and an increase in intracellular glial pH. Ultimately, changes in neuronal and glial interactions with the extracellular space during ictal events end up decreasing [
induces carbonic anhydrase activation, which catalyzes carbonate hydration. The end result of this process is a lower [H+]i and an increase in intracellular glial pH. Ultimately, changes in neuronal and glial interactions with the extracellular space during ictal events end up decreasing [ ]o. This activates carbonic anhydrase, which catalyzes the conversion of carbonate in
]o. This activates carbonic anhydrase, which catalyzes the conversion of carbonate in  and H+, leading to extracellular acidification.
and H+, leading to extracellular acidification.
In the interictal period (figure 8(C)) occurs the recovery of the transmembrane gradients of K+, Na+ and Cl−. The magnitude of such changes is enough to reverse the neuronal HCE exchanger and culminate with  outflow. The recovery of [
outflow. The recovery of [ ]o to baseline levels end up reducing carbonic anhydrase activity. This reduction ultimately allows the extracellular recovery of H+, also to its baseline. In addition, a reduction in the neuronal Na+ concentration decreases NHE activity, which in turn leads to an increase in neuronal pH. Although such reduction in NHE activity would favor an extracellular accumulation of H+, this effect is less pronounced than the reduction of carbonic anhydrase activation. As a result, the final consequence of these events is an increase in extracellular pH. While extracellular volume changes amplify this process, electrodiffusion attenuates such increments in extracellular pH. In glial cells, the main effect of ionic changes is to reverse the HCE exchange. The consequences of such an effect are a reduction in intracellular
]o to baseline levels end up reducing carbonic anhydrase activity. This reduction ultimately allows the extracellular recovery of H+, also to its baseline. In addition, a reduction in the neuronal Na+ concentration decreases NHE activity, which in turn leads to an increase in neuronal pH. Although such reduction in NHE activity would favor an extracellular accumulation of H+, this effect is less pronounced than the reduction of carbonic anhydrase activation. As a result, the final consequence of these events is an increase in extracellular pH. While extracellular volume changes amplify this process, electrodiffusion attenuates such increments in extracellular pH. In glial cells, the main effect of ionic changes is to reverse the HCE exchange. The consequences of such an effect are a reduction in intracellular  and the consequent reversion reaction catalyzed by carbonic anhydrase. This promotes an intracellular augmentation of H+ and, therefore, glial acidification, effects that are further enhanced by the reduction of glial volume.
and the consequent reversion reaction catalyzed by carbonic anhydrase. This promotes an intracellular augmentation of H+ and, therefore, glial acidification, effects that are further enhanced by the reduction of glial volume.
5. Conclusion
We propose that during NEA, changes in pH occur as a result of the combined action of the NHE, HCE, H+ pump and the catalytic activity of intra and extracellular carbonic anhydrase. Cellular volume changes and extracellular electrodiffusion are also responsible for modulating pH changes. The activation of all these mechanisms is associated with intense fluctuations in intra and extracellular concentrations of Na+, K+ and Cl−, which is one of the mechanisms responsible for the intense neuronal activity during the NEA.
Acknowledgments
This work was supported by the Brazilian agencies FAPEMIG, FAPESP, CNPq, PROCAD/CAPES, CAPES and INCT of Translational Neuroscience (MCT/CNPq/FAPESP), MCTI/FINEP/MS/SCTIE/DECIIS—CT—SAÚDE E FNS—1264/13.