Abstract
Electroencephalography (EEG) holds promise as a neuroimaging technology that can be used to understand how the human brain functions in real-world, operational settings while individuals move freely in perceptually-rich environments. In recent years, several EEG systems have been developed that aim to increase the usability of the neuroimaging technology in real-world settings. Here, the usability of three wireless EEG systems from different companies are compared to a conventional wired EEG system, BioSemi's ActiveTwo, which serves as an established laboratory-grade 'gold standard' baseline. The wireless systems compared include Advanced Brain Monitoring's B-Alert X10, Emotiv Systems' EPOC and the 2009 version of QUASAR's Dry Sensor Interface 10–20. The design of each wireless system is discussed in relation to its impact on the system's usability as a potential real-world neuroimaging system. Evaluations are based on having participants complete a series of cognitive tasks while wearing each of the EEG acquisition systems. This report focuses on the system design, usability factors and participant comfort issues that arise during the experimental sessions. In particular, the EEG systems are assessed on five design elements: adaptability of the system for differing head sizes, subject comfort and preference, variance in scalp locations for the recording electrodes, stability of the electrical connection between the scalp and electrode, and timing integration between the EEG system, the stimulus presentation computer and other external events.
Export citation and abstract BibTeX RIS

Content from this work may be used under the terms of the Creative Commons Attribution 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Background
Electroencephalography (EEG) has been used for the last century to study how brain function relates to human performance, and this method is widespread among neuroscience laboratories. The technique uses electrodes on the scalp to record electrical fields generated by neuronal dynamics, which can be analyzed to examine how particular cognitive tasks modulate brain activity. With its small form factor, EEG holds promise as a neuroimaging technology that can be used to understand how the human brain functions in real-world, operational settings while individuals move freely in perceptually-rich environments. Development of advanced technologies of this manner has been emphasized as a high priority [1–3], as they will impact a wide range of applications from enhancing bench level neuroscience efforts [4–7] to optimizing soldier-system design [8–10].
EEG is among the least invasive neuroimaging technologies and features excellent resolution of neural activity in the time domain, although it lacks the degree of spatial resolution possible with other neuroimaging technologies. Recent advances in signal detection algorithms (e.g., [11]) and wireless transmission [12] have led to the development of numerous wireless, multi-channel EEG systems designed to eliminate restrictions on the participant's behavior while decreasing the difficulty of system use and maintenance. These advancements have greatly streamlined the process for acquiring data and demonstrate promise for conducting studies in less restricted environments, complimenting recent research illustrating the gains to be made in this area [13–17].
To date, however, the focus of discussion amongst the EEG technical development community has been on the performance of primary system components, and evaluating the efficacy of new approaches for acquiring data. Examples include advocating the use of 'active' sensors to maximize the SNR, use of dry, gel-free [18–20] or non-contact sensors [21, 22], or simply comparing the signal quality of a whole new system [23–25]. In contrast, there has been very little objective discussion dedicated to the physical features of the system that mitigate the general usability of the system, such as the comfort necessary to wear the system for long periods of time or adaptability to a wide range of head sizes. System usability features have a direct impact on the total system design and its viability for adoption by both consumers and scientists. Consequently, a detailed comparison of recently developed wireless EEG systems to a laboratory-grade system will address pragmatic concerns that benefit the neural engineering community working to develop real-world neuroimaging systems.
In this study, 16 volunteer participants performed the same seven cognitive tasks during four separate experimental sessions in the lab. Participants wore a different EEG system for each of the four 60 m sessions: three commercially-oriented, wireless EEG systems and a wired, laboratory-grade BioSemi ActiveTwo system (figure 1). In addition to comparing the signal quality among the systems [26], we investigated usability features of each system and evaluated their viability for future system development efforts. The system evaluations in this report integrate comments from two populations—feedback from 16 experimental participants who wore each system and feedback from 11 experimenters who oversaw the data collection and analysis of the experiment. Importantly, the amount of previous EEG experience varied across the experimenters, ranging from less than a year to over 15 yr; consequently, this range of experience captures usability comments based on conventions from established neuroimaging laboratories, as well as concerns from relatively novice users learning how to use a new system quickly and effectively. These evaluations highlight several issues related to the general usability of an EEG system, and they have been organized into five main design elements: (1) the adaptability of the system for differing head sizes, (2) the participant's rating of comfort and preference, (3) variance in the selected scalp locations for the recording electrodes, (4) the stability of the electrical connection between the scalp and electrode, and (5) the integration between the EEG system and event timing information for the cognitive tasks from the stimulus presentation computer and other external events. Additional hardware details and related timing accuracy of each system has been discussed previously [27, 28].
Figure 1. Pictures of the four EEG acquisition systems used in this study. Top left: BioSemi's ActiveTwo system was used as the laboratory-grade system for comparison and features a stretchable cap with snap-in electrodes and conductive gel. Three wireless systems were tested. Top right: Advanced Brain Monitoring's B-Alert X10 features a plastic strip with stick-on pads and conductive gel. Bottom left: QUASAR's HMS system features dry electrodes on pivoting arms. Bottom right: Emotiv's EPOC system features saline-soaked felt pads on electrodes at the end of semi-rigid plastic arms.
Download figure:
Standard image High-resolution image2. Descriptions of the EEG systems
The technical descriptions contained here are intended to provide an overview of the four EEG systems used in this comparison study: ActiveTwo from BioSemi, B-Alert X10 from Advanced Brain Monitoring (ABM), the EPOC from Emotiv Systems, and the 2009 helmet-mounted version of the Dry Sensor Interface (DSI) 10–20 from QUASAR (which we refer to as 'HMS') (table 1). The overview section for each system provides details about electrode sensors and their locations, their native recording reference, and the scalp application method. These descriptions specify how each system was configured for use in our laboratory, including technical information about system adjustments for head size variability and acquisition options for collecting and synchronizing the EEG data.
Table 1. General properties of the EEG electrodes.
| System | Electrodes | Electrode locations | Electrode type | Scalp to electrode connection | Reference locations | Scalp area per electrode |
|---|---|---|---|---|---|---|
| ActiveTwo | 64 | 10–10 positions | Active | Gel | Near Pz | Variable |
| B-Alert X10 | 9 | F3, Fz, F4, C3, Cz, C4, P3, POz, P4 | Passive | Gel-infused foam | External | 1.77 cm2 |
| EPOC | 14 | AF3, AF4, F7, F3, F4, F8, FC5, FC6, T7, T8, P7, P8, O1, O2 | Passive | Saline-infused felt | Mastoids and/or P3/P4 | 1.13 cm2 |
| HMS | 9 | F3, Fz, F4, C3, Cz, C4, P3, Pz, P4 | Passive | Direct contact | P4, Fpz | 3.80 cm2 |
2.1. ActiveTwo (BioSemi)—baseline for comparison
BioSemi products are frequently used for EEG research within the neuroscience community, and as such, this system served as an example of a laboratory standard to evaluate the three newer systems marketed to enabling neuroscience in the real world. The ActiveTwo system is capable of simultaneously recording scalp electrical activity from up to 256 electrodes, but for this study, we used the 64 electrodes version which utilizes the standard 10–20 electrode grid scheme (table 1). Additional channels are available and can be placed anywhere according to the researcher's requirements. We recorded from two sites on the right and left mastoid to be used as a reference as this matches previous studies from our laboratory (e.g. [29–31]).
The ActiveTwo system is unique compared to most other systems in that it natively records data without reference, requiring it to be manually subtracted later during processing. The system is also capable of receiving external event code triggers through a single 16 bit interface (two 8-bit parallel ports) using an external integration box (part of the ActiveTwo system) which interfaces directly with the acquisition hardware prior to reception with the PC.
To place the ActiveTwo system electrodes on the head, a snug fitting cap with electrode holders is first stretched over the head. The experimenter then inserts a conductive gel at each electrode site to fill the gap between the scalp and electrode and then snaps the electrodes into the cap. Although the gel results in an easily established and relatively stable electrical connection through non-conductive hair, it takes time to setup and clean the equipment. The manufacturer sells the electrode caps in sizes ranging from extra small to extra large to accommodate differing head sizes, with caps ranging from 46 cm to 66 cm diameter (http://www.Biosemi.com/headcap.htm). Most of our study participants used the medium/large head cap that fits heads with a circumference between 56 and 60 cm. The ActiveTwo system is wired, so the participant must be physically tethered to a data collection computer. The complementary data collection software provided by the manufacturer, 'ActiveView,' is a LabVIEW (National Instruments , Austin, TX) based script, and as such, it is easily manipulated to integrate external signals from other sources (such as a PC parallel port, serial port, TCP/IP, etc). The native hardware is capable of receiving external event code triggers from stimulus presentation software through a single 16 bit interface (which can be paired to up to two 8-bit parallel ports) using an external integration box which interfaces directly with the acquisition hardware prior to reception with the PC.
2.2. B-Alert X10 (ABM)
Currently, ABM makes an EEG head unit (X24) with 20 electrodes; however, at the time of testing, we used the B-Alert X10 that consists of a flexible plastic strip connecting nine electrode sites that are located on the standard 10–20 electrode grid at F3, Fz, F4, C3, Cz, C4, P3, POz, and P4 (table 1). Two additional, separate but electrically-linked electrodes connect to the head unit via wires to provide the signal reference, which we placed on the right and left mastoids to match our configuration of the ActiveTwo comparison system. The reference electrode leads, which require minimal gel, attach to the skin via a standard adhesive pad similar to most medical grade pads. The EEG electrodes embedded within the flexible plastic strip are flush with the surface of the plastic. Adhesive sponge discs are attached to the electrode surface to enable gel to be applied inside a hollow center ('donut hole') of the adhesive sponge to lower impedance and increase conductivity between the scalp and each electrode. The system also includes two additional, optional leads for recording electrocardiography, which were applied to the left sternoclavicular notch and the right fifth intercostal rib space. This unit has multiple sizes of electrode strips to accommodate different head sizes; the small and medium sizes of the electrode strips accommodated the head sizes of our study participants. This unit is capable of wired or wireless (Bluetooth) transmission to the data collection computer, and we acquired EEG data in the wireless mode for this comparison study.
The manufacturer-supplied software package supports integration with secondary sources for event triggering through a parallel port, serial port or a TCP/IP connection. In addition, the manufacturer also supplies a C++ SDK and MATLAB routines compiled in C for communication directly with the headset, so the user can develop their own software if desired. The B-Alert X10 system permits event code triggers via serial or parallel port directly at the wireless receiver. Consequently, these signals are integrated prior to communicating with the acquisition PC.
2.3. EPOC (Emotiv)
The EPOC head unit consists of 14 data electrodes placed at pre-designated 10–20 electrode locations: AF3, AF4, F7, F3, F4, F8, FC5, FC6, T7, T8, P7, P8, O1, and O2 (see table 1), Electrodes used for reference and common-mode sensing are hardwired in parallel to locations that approximate the P3/P4 locations and the two mastoids, such that users select their preferred reference locations (e.g. use either P3 and P4, or left/right mastoids) and place rubber non-conductive pad in the alternative location. We chose the mastoid locations to match our configuration of the ActiveTwo comparison system, placing rubber pads at the P3/P4 locations. Gold-plated, passive electrodes contact the scalp through saline-soaked felt pads. The headset also includes two accelerometers for measuring head movement. This system has a limited ability to accommodate differing head sizes, because the electrodes are mounted on semi-rigid plastic arms connected to a plastic frame that wraps around the head and also contains the battery/transmitter unit. The flexible arms that house the electrodes can be adjusted to small distances, but overall, the unit does not provide much accommodation for very small or very large head sizes. The EPOC system only permits wireless communication between the headset and a USB data collection device via a proprietary 2.4 GHz protocol. Some initial signal conditioning (filtering, resampling, etc) occurs on the headset prior to transmission. The system does not provide means for additional external trigger integration prior to acquisition by the primary PC, and it relies solely on software-based integration by the host PC. The native data acquisition software 'TestBench' does provide an option for receiving external events through the PC serial port, although it should be noted that in separate testing we found substantial timing jitter and signal drift with this method [27, 28], An SDK is available from the manufacturer for the headset, potentially allowing the user to develop their own software to integrate signal from other sources if desired. Some MATLAB routines are also available through third parties although they are not currently developed by the manufacturer directly.
2.4. HMS (QUASAR)
Currently, QUASAR makes an EEG head unit (DSI 10–20) with 21 electrodes that record in aggregate and contact the scalp directly. At the time of testing, we used a prototype version designed to fit within an Army combat helmet with only nine electrodes that are positioned at the nominal F3, Fz, F4, C3, Cz, C4, P3, Pz, and P4 10–20 electrode locations [32]. We refer to this helmet-mounted system, which we used without the external helmet shell, as 'HMS.' The electrode at P4 is predefined as the reference electrode and a ground electrode is built into the chin strap. There are no options for recording mastoid or ear location as a reference, so this system cannot match the reference site configuration of the ActiveTwo comparison system. The system also includes an EKG chest belt but it was not used in this study due to technical issues related to securing the battery and setting the data transfer mode. The system is 'dry' because it requires no gel or fluids on the scalp and it does not use any consumable supplies to collect data. The dry electrodes, which consist of an array of spring-loaded metallic pins, rely on direct contact with the scalp. Each sensor has a notch for inserting a tool that can twist the sensor back and forth to separate hair and facilitate direct contact between the sensor and the scalp, a process which is essential to successfully record scalp electrical activity from these electrodes. The electrodes are connected to and spaced around the electrode at Cz by rigid, hinged bars, so this unit has a very limited ability to accommodate differing head sizes. This unit can be used in both a wired and wireless (2.4 GHz proprietary protocol) mode. To support wireless data transfer, a wireless transmitter and replaceable battery can be attached to the back of the head unit. For the system used in this study, the only hardware option for external event code trigger integration from separate stimulus presentation software is via a single binary connection to the wireless receiver. In the system that we used, the native data collection software, 'Q-Streamer,' creates a file with event latencies without event codes. This necessitates a third-party file generated by the stimulus presentation software which must be manually synchronized and integrated with the binary file generated by the manufacturer's data collection software (see [27] for a full description of the correction routine). We acknowledge that additional options for synchronizing event information have evolved since the time of data collection in this study.
3. Tasks and hardware configuration
3.1. Participants and tasks
Sixteen right-handed participants (seven females) between the ages of 19 and 45 volunteered to be fitted with each of the four EEG acquisition systems on four different days. During each 60 min experimental session, the participant performed seven cognitive tasks that cover a range of common experiments. These included visual processing tasks with and without imposed radio frequency artifacts, working memory tasks ('N-Back') with two levels of difficulty, an error-monitoring task, and an artifact inducing task which required participants to perform several specific muscular actions such as blinking and jaw clenching [33]. Neuroimaging data from these tasks are not discussed since they have been described in part elsewhere [26], and this report focuses on issues related to general usability of the different EEG systems, not the signal quality. The order in which the participants experienced the EEG systems was counter-balanced, but the task order remained constant in order to compare the systems, not the tasks. At the end of each experimental session, each participant provided feedback about their experience by discussing their level of comfort during the experiment. Experimenters also made notes about their experiences, such as how long it took to set up each subject, any problems that occurred, or complaints from subjects. After wearing all four systems, participants ranked them in order of comfort and preference. All participants provided written informed consent in accordance with procedures approved by the Institutional Review Board of the US Army Research Laboratory, and all testing conformed to the guidelines set forth by the 1964 Declaration of Helsinki.
3.2. EEG acquisition
The respective acquisition software for each EEG system was run on a Dell Precision 690 running Windows XP SP3. All of the EEG receivers (wired and wireless) were connected to this PC via USB. Task stimuli were presented with E-Prime 2.0 on a separate Del Optiplex GX620 running Windows XP SP3. Stimulus events were also logged on the data acquisition computer, though the method of data transfer varied among systems due to differences in capabilities (see table 2).
Table 2. General properties of the EEG systems.
| System | Manufacturer | Sampling rate (Hz) | Bandwidth (Hz) | Wireless transmission | Accelerometer | Trigger inputs |
|---|---|---|---|---|---|---|
| ActiveTwo | Biosemi | 2048 or greater | User defined | None | No | Parallel port |
| B-Alert X10 | ABM | 256 | 0.1–100 | Bluetooth | No | Parallel port |
| EPOC | Emotiv | 128 | 0.2–43 | Proprietary wireless | Yes | Serial port |
| HMS | QUASAR | 240 | 0.02–120 | Proprietary wireless | No | Single TTL |
pt?>Although we implemented procedures to acquire data under as similar conditions as pragmatically possible, there were some differences among the setup of each system. Our approach relies on equating electrode position across systems using the standard 10–20 electrode grid locations. Consequently, to maximize the spatial alignment of the electrodes across the recording sessions, the central Cz electrode was placed at the intersection of two measurements: the center point of an auricle to auricle measurement and the center point of a nasion to inion measurement. However, despite this procedure, variability in electrode placement occurred among the systems (discussed in section 4.3). As noted earlier, only three of the four systems could use the right and left mastoid reference since the HMS predefines the electrode at P4 as the reference. Furthermore, the systems also require different interactions between the subject and experimenter. The EPOC, B-Alert X-10, and HMS systems are initially adjusted by the subject with minor adjustments in electrode placement made by the experimenter while the ActiveTwo requires that the experimenter perform all of the system preparation with only minor adjustments by the subject. None of these inconsistencies among the system configurations are expected to negatively influence the interpretation of the usability findings from this comparison study.
4. Issues affecting system usability
Replicating a single experiment with four different EEG acquisition systems gave participants and experimenters an opportunity to compare directly the pros and cons of using three new wireless EEG systems in comparison with a wired, laboratory-grade system. We focus the current discussion to five main design elements of the tested EEG systems: adaptability of the system for differing head sizes (4.1), participant ratings of system preference and comfort (4.2), differences in available scalp locations for the recording electrodes (4.3), stability of the electrical connection between the scalp and electrode (4.4), and integration between the EEG system and event timing information for the cognitive tasks from the stimulus presentation computer (4.5).
4.1. System adjustments for head size
Variability in the size and shape of the human head must be considered when designing an EEG system intended for use by the general population. Our baseline laboratory-grade system, the ActiveTwo, accommodates this variation by creating a range of sizes for their electrode caps, a technique common for traditional laboratory EEG systems. Their extra small, adult electrode cap fits a head circumference between 46 and 50 cm, while their extra large cap fits a head circumference between 62 and 66 cm (http://www.Biosemi.com/headcap.htm). As is discussed in section 4.3, these stretchable caps ensure electrode positions at standard or modified 10–20 locations after setting a few reference points. This requires that a laboratory purchase and maintain several different size caps for a given study, but it allows for a degree of subject-specific customization. None of the other three tested systems support this degree of adjustment for differing head sizes and shapes among study participants.
The B-Alert X10 system does have different sizes for the plastic strip that holds the electrodes on the scalp. These strips can account for some variability in the length and width of the head, but not for the variability in the shape or roundness of the head without also adversely affecting electrode placement (see section 4.3). The EPOC and HMS share an alternative, but similar design, where the electrodes radiate out from specific locations and attempt to accommodate longer and wider heads with flexible components. The EPOC utilizes semi-rigid arms that can flex outward for larger heads; however, some electrodes do not contact the scalp in very small heads. In contrast, the HMS has a complex system of spring-loaded joints on arms radiating out from the central (Cz) location which allow for a range of motion to the each electrode, so it can accommodate most adults. However, the small heads of children and adults with extremely large heads are largely incompatible. This additional complexity also creates additional potential burdens for repair and maintenance of the head unit.
Maintaining the position of the EEG electrodes on the head is very important to obtain a stable and reliable signal. The ActiveTwo cap is relatively stable on the head, as long as the participant does not scratch their scalp or otherwise adjust the cap during the recording session; in contrast, the B-Alert X10, EPOC, and HMS systems are not as snug to the scalp and can move around if there is sufficient movement by the user. Although the current comparison study did not require large-scale, continuous body movement, this instability in system position will be an issue for stability of recording sites in future systems designed for use in real-world, operational settings while individuals move freely in perceptually-rich environments.
4.2. System preference and comfort ratings
4.2.1. Statistics
Twelve of sixteen participants provided overall preference rating data for all four of the systems, ranking their most-preferred system as a one and their least-preferred system as a four. Table 3 shows rank descriptive scores for each system, omitting any participants that did not complete reports for all cases.
Table 3. Descriptive statistics of subjects' overall preferences.
| Percentiles | ||||||
|---|---|---|---|---|---|---|
| System | N | Minimum | Maximum | 25th | 50th (median) | 75th |
| B-Alert X10 | 12 | 1.0 | 2.0 | 1.0 | 1.0 | 2.0 |
| ActiveTwo | 12 | 1.0 | 4.0 | 2.0 | 2.0 | 3.0 |
| EPOC | 12 | 1.0 | 4.0 | 3.0 | 3.0 | 4.0 |
| HMS | 12 | 1.0 | 4.0 | 2.3 | 4.0 | 4.0 |
The subjective ratings, based on an ordinal scale, were analyzed using a non-parametric Friedman's test with Wilcoxon sign-rank tests for a priori pair-wise comparisons between BioSemi and each of the other systems (table 4). A statistically significant difference in ratings was found among the systems overall (χ2(3) = 16.5, p = 001; Kendall's W = 0.458). Follow up comparisons reveal that the B-Alert X10 was preferred significantly more often than the ActiveTwo system (table 4; z = −2.44, p < 05). The rating comparisons between the other systems suggest that subjects generally preferred the ActiveTwo more often than the EPOC and HMS, although these differences fell short of the criterion for significance. Across the group, all of the systems received rankings ranging from being the most-preferred (1) and least-preferred (4), except for the B-Alert X10 which was ranked by all participants in the top two slots, reflecting the overall comfort of the system.
Table 4. Comparative statistics of subjects' overall preferences.
| System comparison | Z score | p value |
|---|---|---|
| X10–ActiveTwo | −2.44 | 0.015 |
| EPOC–ActiveTwo | 1.57 | 0.118 |
| HMS–ActiveTwo | 1.77 | 0.076 |
Additionally, thirteen of the sixteen participants provided comfort ratings for all four systems on a scale from 1 to 7, with 1 labeled as 'very comfortable' and 7 labeled as 'very uncomfortable' table 5 shows the descriptive comfort rank scores for each system. Similar to the ranking data shown in table 3, the variability between the minimum and maximum ratings was smallest for the B-Alert X10 system (1–4) reflecting the overall comfort of the system, although the HMS also showed a slightly tighter range (1–6) than the ActiveTwo and EPOC systems (1–7).
Table 5. Descriptive statistics of subjects' comfort ratings.
| Percentiles | ||||||
|---|---|---|---|---|---|---|
| System | N | Minimum | Maximum | 25th | 50th (median) | 75th |
| B-Alert X10 | 13 | 1.0 | 4.0 | 1.5 | 2.0 | 2.5 |
| ActiveTwo | 13 | 1.0 | 7.0 | 2.0 | 3.0 | 4.5 |
| EPOC | 13 | 1.0 | 7.0 | 4.0 | 5.0 | 6.0 |
| HMS | 13 | 1.0 | 6.0 | 3.5 | 5.0 | 6.0 |
A Friedman's test indicated overall significant differences in comfort ratings between systems, (χ2(3) = 20.52, p < 001; Kendall's W = 0.526). Follow up comparisons showed comfort ratings between the B-Alert X10 and the ActiveTwo were significantly different indicating more comfort for the X10 system (z = −2.35, p < 05), while the HMS was rated as significantly less comfortable than ActiveTwo (z = 1.97, p < 05) and there was no significant difference with the EPOC system (table 6). For comparisons of both preference and comfort, including partial data did not substantially alter the results.
Table 6. Comparative statistics of subjects' comfort ratings.
| System comparison | Z score | p value |
|---|---|---|
| X10–ActiveTwo | −2.35 | 0.019 |
| EPOC–ActiveTwo | 1.56 | 0.12 |
| HMS–ActiveTwo | 1.97 | 0.049 |
4.2.2. General observations
Overall, concerns about system comfort identified by participants can be grouped into three broad categories: discomfort due to pressure on specific points of the scalp, discomfort from overall weight or constriction of the unit as a whole, and difficulty of general application and clean up.
When a head unit does not fit correctly, pressure may not be distributed across the area as designed, resulting in discomfort for the subject. For the laboratory-grade ActiveTwo system, discomfort typically occurs with a cap that is too small. Over an extended period of wear, this constant pressure may result in subject discomfort. However, this is a rare problem because researchers can select an appropriately sized cap for the user from a wide range available from the manufacturer. For the EPOC and HMS systems, however, the electrode array is held in place by spring-loaded pressure. On the EPOC, these are plastic pre-bent arms, while the HMS headset uses spring-loaded metal arms. In both cases, subjects reported feeling 'hot spots' that developed over the hour long experimental session due to the pressure of the electrode pad. Generally, these types of complaints did not appear immediately, but rather after several minutes or even an hour of wearing time. Also, problems of this nature were mostly common with individuals with larger heads, due to the increased pressure created by the spring action (especially the Emotiv system—see also section 4.3). We did not receive any similar complaints about the ABM B-Alert X10, likely because it uses relatively little pressure at the electrode site to hold it in place.
The ActiveTwo system components worn on the head are not very heavy (170 g), but both the B-Alert X10 and EPOC are notably lighter (110 and 125 g, respectively). The HMS, in contrast, headset is considerably heavier (510 g) and bulkier than the other units. The heavy HMS head unit resulted in some reports of neck pain and headaches by participants towards the end of the experimental session. This lack of comfort may arise because the weight is focused on fewer locations on the scalp rather than being distributed across the scalp in a diffuse number of points, as with the conventional caps used in the ActiveTwo. Multiple participants noted that the HMS was comparatively heavier, and one specifically complained about discomfort due to the system weight. The EPOC system also elicited similar problems stemming from uneven weight distribution, as it has fewer points of contact with the scalp, while no such complaint arose with the B-Alert X10 head unit. For future systems, determination of a comfortable, maximum weight for an EEG head unit will vary across participants, but designs that minimize pressure points are likely to be more comfortable overall.
Finally, we were surprised at participant feedback regarding system application and clean-up. While participants clearly would prefer minimal gel in their hair, their overall concerns were biased less towards this factor than towards the general comfort of use and ease of application; this is reflected in the ratings of overall preference described above (tables 3 and 4). For example, many noted how simple and non-invasive the saline-based EPOC system was; however, participants who had a lack of comfort from EPOC pressure points found this to be a greater problem than the gel used by the B-Alert X10 or ActiveTwo systems. Likewise, although the HMS system required virtually no clean-up and only minimal preparation from subjects, the difficulties encountered in twisting the electrodes to achieve a good signal (see section 4.4) and pressure from the electrode pins outweighed this advantage. This is probably why participants had an overall preference for the B-Alert X10 system. Even though it required some gel, the cleanup was minimal (especially when compared to ActiveTwo), and it was lightweight with virtually no problems of comfort, even for participants with larger head sizes who were included in this study.
4.3. Variability in electrode locations
EEG acquisition device manufacturers typically describe the position of their sensors in the context of the International 10–20 system for electrode placement [34, 35] (table 1). Even though this system is not perfect [36], it is essential for comparison of brain activity measurements taken in different labs, which inevitably entails different subjects, equipment, and approaches to electrode placement. Each of the four systems discussed in this manuscript features a different form factor for fitting electrodes to the scalp, which will have an influence on the precision and accuracy of electrode placement. We measured the actual electrode locations on a total of ten people wearing all four systems, and compared these data to the ideal target 10–20 locations for the wearer.
4.3.1. Impact of form factor on electrode placement
A major challenge in comparing data across different EEG systems is the inconsistency of electrode locations. Although each system states the 10–20 locations for the electrodes (the industry standard scheme based on an inter-electrode percentage relative to the total scalp distance; see table 1), the accuracy of these intended locations during an EEG recording session may vary based on how the EEG systems fit across different head sizes and shapes. In the conventional method utilized by the laboratory-grade ActiveTwo system, the caps stretch between electrode locations to distribute them approximately equivalently with the percentage-based inter-electrode distances from subject-to-subject, and a specific cap size is chosen so that the range is correct. However, this requires purchasing and maintaining multiple caps. In contrast to this approach, the EPOC and HMS systems target a one-size-fits-all approach by utilizing semi-rigid components that allow the electrodes some flexibility in fitting different head sizes; however, the inter-electrode distance (for some electrodes) is fixed, limiting the total compensation possible for inter-electrode distances. The B-Alert X10, alternatively, comes in a range of sizes of electrode strips with a fixed distance between electrodes and attachment points at the end of the strips to an elastic headband.
In all of these systems, the manufacturer provides instructions for placement of the system on the wearer's head based on aligning one or two electrodes to anatomical landmarks (ActiveTwo: Cz; B-Alert X10: Cz; EPOC: T7/T8; HMS: Cz). The user must then accept a certain error in placement of the rest of the electrodes. For the ActiveTwo, the most common solution to this problem is to purchase a variety of cap sizes so that the error is minimized for each subject. For the EPOC, the fixed curvilinear distance between T7/T8 and the other electrode sites cannot be adjusted. Similarly, the HMS electrodes radiate out from CZ, which results in varying 10–20 location errors, highlighting the same fundamental issue for EEG systems with fixed inter-electrode distances. The B-Alert X10 allows setting the position of a single electrode site, but the inter-electrode distances are fixed along the plastic strip. The strips are pulled tight and fastened with Velcro to the headband in order to maintain good connectivity, which allows for some judgment in electrode placement when the system is put on the wearer. However, it should be noted that the plastic strip yielded a unique issue where it buckled in the back, with the plastic strip having to fold and bunch in order for the connector to align properly, resulting in some connectivity problems with the electrodes near the back of the head.
Another critical variation among the systems is the scalp areas covered by the electrodes (figure 2). Most notable in the coverage differences, the EPOC system lacks recording locations along the midline. When selecting a system for real-world applications, EEG experiments should consider which scalp locations produce the strongest signal of interest and take care to select an EEG system that will provide consistent and sufficient coverage of these scalp areas across participants.
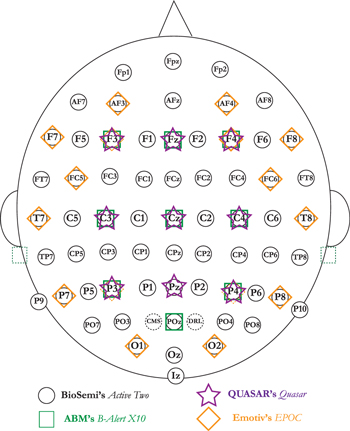
Figure 2. Manufacturer's stated electrode positions. BioSemi's ActiveTwo had 64 recording locations with a recording reference located near POz and additional recording electrodes placed at the mastoids. ABM's B-Alert X10 uses nine recording locations and reference the electrodes located at the mastoids. QUASAR's HMS system has nine recording electrodes, reference predefined as electrode at location P4, and a ground electrode on the chin (not shown). Emotiv's EPOC has 14 recording locations and hardwired references located at P3/P4 or left and right mastoids (user's choice).
Download figure:
Standard image High-resolution image4.3.2. Measurement of variability in electrode locations
To determine the extent of the variability in electrode placement between systems, we digitized electrode placement of each system on ten different individuals with an ELPOS system (Zebris; Isny, Germany). This generates polar coordinates (azimuth, latitude, and radius) for each electrode position relative to anatomical points. These coordinates are compared to the actual 10–20 coordinates projected onto a sphere using the radius measured at that location, yielding differences in cord length between points (error in mm). Error reported here thus represents the accuracy and inter-individual variability in electrode placement for each system relative to the manufacturer's stated position. For the purpose of this study, we used the width of the plastic electrode snap for the ActiveTwo system. Based on indentations and residue left on subjects' skin after wearing the ActiveTwo, it is rare that gel extends beyond this area.
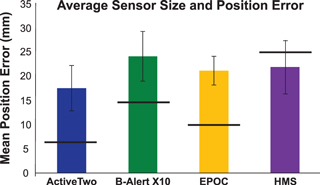
We found that the average error in the placement of the center of the electrode was comparable for all four systems (figure 3). However, if the size of the sensor is taken into consideration (see the horizontal bars in figure 3), the HMS system is the most accurate due to the large diameter of the sensors. It should be noted that for all systems, including the ActiveTwo, the size of the scalp area covered by the electrode compensates for the imprecision in the 10–20 location.
Figure 3. The average distance between the measured electrode positions and 10–20 positions for each system (error bars show standard deviation across subjects). Horizontal bar marks the diameter of the sensor for each system.
Download figure:
Standard image High-resolution imageThe three wireless systems showed imprecision at specific regions of the scalp based on the form factor of the head unit. Figure 4 plots this error for each electrode in relation to the laboratory-grade ActiveTwo system. We found that the B-Alert X10 repeatedly showed errors and inter-individual variability at position POz. This is likely a result of how the cap is bent in order to plug into the electronics unit (see section 4.3). Otherwise, the variability of the B-Alert X10 is similar to variability in the ActiveTwo system. The EPOC shows the highest amount of error at O1 and O2 at locations at the back of the head over occipital cortex and F3 and F4 at locations at front of the head over frontal cortex. These positions are most susceptible to location errors based on the fixed length of the semi-rigid arms and variability in head size that changes the distance between these arms when expanded to fit the head circumference. The HMS shows the highest amount of position error for the P3 and P4 electrodes positioned over the parietal cortex. However, since the HMS has relatively large electrodes, collecting potential across a broad surface area, the position offset is likely least relevant for this system.
Figure 4. Each plot shows the average variability of electrode location across subjects for each of the three tested wireless EEG systems in comparison with the variability in location for the laboratory-grade ActiveTwo system. Each red bar indicates the median, with the box defining the 25th and 75th percentiles and whiskers extend to the most extreme datum not considered 'outliers', which are marked by a red asterisk. The y-axis plots the distance in millimeters of each electrode position in the four EEG system head units from the polar coordinates defined by the standardized 10/20 electrode positions using a Zebris ELPOS digitization system (see section 4.3.2). The x-axis plots the electrodes specific to each of the three tested wireless systems. The data for the ActiveTwo system are shown in blue, and data for ABM's B-Alert X10 shown in green (top), Emotiv's EPOC shown in yellow (middle), and Quasar's HMS in purple (bottom).
Download figure:
Standard image High-resolution image4.4. Electrical connection styles, setup time and stability
4.4.1. Styles and setup
Across the four systems, three approaches were implemented to facilitate conductivity between the electrodes and the scalp. The first was electrically conductive gel, which is the traditional approach for laboratory EEG systems used by the ActiveTwo and B-Alert X10 systems. This approach allows for electrical contact through hair, but increases set up time to gel each electrode (64 for ActiveTwo and 9 for X10) and requires clean up time for the participant at the end of the EEG recording session. The application of the gel is accomplished more quickly using the ABM design because there are only nine channels, and each electrode has the gel applied prior to application to the participant's head. Thus, the experimenter only needs to push down on the electrode once it has been placed on the participant's head to release the gel. The BioSemi system requires that a syringe of gel be used to fill each electrode separately after the EEG cap is positioned on the participant's scalp, which is time consuming (taking two experimenters working concurrently around 20 m to gel 64 electrodes).
The second approach utilized by the EPOC system was saline-soaked foam pads, which is also capable of establishing a connection through most types of hair. The pads are soaked in saline before they are inserted in the headset, so there is nothing to apply after the headset is positioned on the participant's scalp. This approach is also advantageous to the participant because the only residue left in their hair is saline. Since saline is roughly similar to sweat, it dries quickly after completion of the session and is not bothersome to the user.
The third approach was 'dry' electrodes on the HMS system, which required no gel or additional fluid. The dry electrodes are designed to work through hair by inserting a tool into a notch on the top of the sensor and twisting the sensor back and forth to separate hair, enabling direct contact between the sensor and the scalp. The pins are spring-loaded on concentric rings so that they roughly conform to the scalp. Thus, the array of electrode pins used by the HMS system is capable of contacting the scalp through hair. However, the HMS electrode can become tangled in long, thick hair resulting in an uncomfortable experience for the wearer and/or insufficient connectivity. We were unable to establish sufficient connectivity with the HMS system in one participant with thick, curly hair, despite success with the other three systems. As a result, although the HMS is more convenient for the participant because no liquid residue remains in their hair, two major trade-offs for the system are not comfortable to the participant from the twisting of the sensor in their hair and additional cleaning by the experimenter to remove tangled hair from the unit.
4.4.2. Total setup time
In addition to the initial application of the system, the total time to prepare the participant was also affected by the effort necessary to obtain acceptable electrode impedance, which indicates the quality of connection with the scalp. For example, with the HMS unit, even though the initial time to setup the headset on the participant was very quick, it often took considerable effort to make strong, reliable contact between the electrodes and the scalp at all positions simultaneously, especially at the Cz location. Additionally, this system required a 'settling time' of several minutes for the impedance to come within an acceptable range after application. As a result, the total time for preparation often matched or exceeded what was typical for the gel-based systems. In contrast, the EPOC and B-Alert X10 systems only required a small amount of manual manipulation of the electrodes to work the conductive liquid into contact with the scalp because the application of saline or conductive gel could be completed prior to the participant's arrival for the study. Thus, based on the current designs, the less dense, wet electrode systems were generally faster to apply than the dry electrode system. The BioSemi ActiveTwo still required the most time, simply due to the substantially larger number of channels to set up.
Another component of the total setup time relates to the ease of reading the current impedance values of each electrode. The interface for each of the three wireless systems was easier to use than the 'ActiveView' software for the laboratory-grade comparison system. In ActiveView, the voltage offset (impedance is not reported, but offset from the common mode follower circuit is used as a surrogate) is shown in a bar plot for all 64 channels on the same screen, so it is often time consuming to decipher which bar on the screen corresponds to which location on the scalp. In contrast, the other three systems report impedance in a graphical depiction that plots each electrode on the scalp, which greatly eases identification. It should be noted, however, that while both the HMS and EPOC software report in real-time, the current software for B-Alert X10 requires a manual button click to begin calculation to compute the impedance for the entire set of channels (taking takes several seconds for each electrode). As a result, the total time to read impedance on all channels for the B-Alert X10 system can be extremely long, and the duration of this process is extended every time a channel is manipulated and must be re-checked. Altogether, it was universally agreed among the experimenters collecting data that while the EPOC took the least amount of time and effort for total setup, and the ActiveTwo cap consistently took the longest, it was unpredictable whether the B-Alert X10 or HMS might be equally time consuming as the substantially higher-density ActiveTwo system.
4.4.3. Contact stability over time
Across all three approaches in the newer systems, we were pleasantly surprised by the stability of the connection between the electrodes and the scalp. Conductive gel (used by both ActiveTwo and B-Alert X-10) dries out a small amount during the 60 min experimental session which can result in an itchy sensation on the scalp for the participant, but it remained conductive throughout the experiment. The saline-soaked felt pads used in the EPOC remained damp enough to be conductive throughout the experiment, although this may be contingent on the relative humidity of the area where they are being used. The dry electrode sensors on the HMS system also maintained their conductivity as long as the headset position was not adjusted by a head scratch or other manipulation at the scalp.
As neuroscience moves from the laboratory into more complex settings, environments that cause a participant to sweat should increase the durability of scalp-electrode connections for every connection type. However, for any fluid conductivity-based system, there is a risk of bridging the gaps between electrode sites in high-density electrode systems. Interestingly, sweat may enhance the signal quality in the pin-array style of dry electrodes by bridging the small inter-pin distances. Excessive sweating, however, may result in an electrical bridge between pin arrays identical to the bridging between electrodes in other systems. Future EEG systems designs will need to be implemented and tested in whole-body-movement paradigms and various temperature environments to examine these real-world recording considerations.
4.5. Event integration
Typically for research purposes, EEG is time-locked to an external event or action to understand the brain's response to a specific activity occurring in the environment. To synchronize the EEG data with experimental tasks, environmental cues, and other sensors, there must be a method for integrating event triggers with the EEG data. The precision and reliability of this synchronization can be critical to properly assessing the neural response, which can vary by only milliseconds. Each of the systems compared utilizes a different method for logging event triggers. Some systems use a parallel data port (ActiveTwo, B-Alert X10) which allows for logging not only the timing of an event, but permits coding of different types of events. This is the most common among conventional laboratory systems, and it is the preferred method because it simplifies the analysis and prevents mislabeling of event types. In fact, the B-Alert X10 receiver has connections for two serial inputs as well, greatly increasing the options available for different devices to synchronize. EPOC offers a method for recording event triggers over the serial port, but the timing is highly susceptible to jitter and delay due to a reliance on the recording PCs operating system to coordinate with the serial port for integration with the EEG data, instead of using a separate integration box (details of this have been described in [27]). The HMS uses a simple TTL pulse to signal that an event occurred, but this does not permit coding of different event types or classes; as a result, this solution requires a post-processing step to decode what event type occurred for each TTL pulse. The timing of data trigger integration has been covered in depth elsewhere [27, 28], but the main conclusion from that work is that the transfer of data into the acquisition PC prior to integration of event triggers negatively impacts the synchronization of EEG data with events of interest. This issue was of particular concern for the EPOC system, which is the only system of the four discussed here that does not use an external method of synchronization.
5. Summary
Recently, several commercial-grade, wireless EEG systems have been developed that aim to increase the usability of the neuroimaging technology in real-world settings. We recruited 16 participants to complete a series of cognitive tasks in four experimental sessions to assess the pros and cons of using three new wireless EEG systems in comparison with a wired, laboratory-grade system. BioSemi's ActiveTwo served as an established laboratory-grade 'gold standard' baseline for a comparison with three wireless EEG systems: the ABM B-Alert X10 system, the Emotiv EPOC system, and the early QUASAR HMS system. The experimental design for this comparison study provided both participants and experimenters an opportunity to directly compare the EEG systems, and the design of each wireless system is discussed in relation to its influence on the system's usability as a potential real-world neuroimaging system.
Only the standard ActiveTwo system has a systematic approach to accommodate variation in both head size and shape. The wireless system with the most comparable performance was B-Alert X10 which uses different sizes for the plastic strip that secures the electrodes and accommodates variable head sizes; however, this solution was less adaptable for a range of head shapes. Meanwhile, the flexible sensor arms on the EPOC and HMS headsets provided the opposite accommodation—some adaptability for head shape, but not for head size. Even worse, the flexible arms resulted in uncomfortable pressure points across participants and undesired movement of the entire unit on the scalp with participant head rotation. Additionally, participant feedback revealed that the B-Alert X10 system was preferred overall compared to the EPOC and HMS systems, and that the B-Alert X10 system was also rated as significantly more comfortable than the ActiveTwo comparison system.
Experimenter feedback identified additional usage concerns based on the variation in the system designs. The intended 10–20 electrode locations of the systems were not accurate across participants because the fixed inter-electrode distances could not accommodate variability in head sizes and shapes. The systems also vary in the coverage of scalp locations, which may be significant for particular tasks if the brain signal of interest occurs most strongly at an uncovered location on the scalp. It is important to note that many manufacturers of wireless EEG systems are actively developing form factors utilizing higher densities of electrodes to accommodate the requirements of signal decomposition analyses. It is unclear whether increasing the sensor density may limit the deviation from actual 10–20 locations by reducing the available space on the scalp. However, the structural sources of error that result from semi-rigid arms, stretchable caps, and fixed-distance systems will remain.
All three wireless systems showed good stability in the signal connectivity over the time period of recording, even though three different approaches were used for establishing contact between the electrodes and the scalp. However, the approaches did differ in their comfort to the user, ease of setup for the experimenter, and total time for setup. The current wet designs out-performed the dry design overall in this respect, even with the clean-up time saved from the lack of gel. Finally, all systems used slightly different methods for event synchronization, with the B-Alert X10 overall providing the largest number of options (both parallel and serial input), and the HMS system having the most limited option (only a single binary timestamp) which made it necessary to rely on additional third party event logging and file synchronization post-hoc (options from the manufacturer have evolved since the time of testing).
This study highlights the need for ergonomic assessments [37] of neurotechnologies, including a need to define quantifiable goals for usability of EEG systems to determine the utility of various design elements for specific neuroscience applications [4, 6, 9, 10, 38]. This report focused on five design elements: (1) adaptability for head size variance, (2) comfort and preference, (3) variance in the selected scalp locations for the recording electrodes, (4) the stability of the electrical connectivity, and (5) the integration between the EEG system and other information. However, within each of these elements, more systematic study of usability factors would enhance our knowledge for future system development efforts. Candidate factors include measurement of discomfort, in addition to comfort, and how these ratings evolve over time since a system may be comfortable for an hour but unbearable for three; recording the time for each of the setup and clean up steps (prepping the cap or participant, establishing impedance levels, etc) to quantify differences among the systems; characterization of hair types and how they influence impedance levels and comfort ratings; additional ergonomic issues, such as sustainability [39], and their impact on usability; and establishing thresholds for specific applications of the system because a system viable for one use, e.g., seated in air conditioning, may not be viable for another use, e.g., walking around a heated desert. Future work is needed to systematically evaluate a host of usability factors tailored to the specifics of the intended neuroscience application.
6. Conclusions and recommendations
Out of the three wireless systems tested, participants and experimenters preferred the approach by ABM on the B-Alert X10. The gel-infused sticky foam pads were easy to apply and comfortable to wear. The saline-soaked pads at the end of semi-rigid 'fingers' (EPOC approach) are designed for easy application and wear, but the semi-rigid 'fingers' result in too much pressure for some larger heads (leading to decreased comfort over time). In contrast to this, the stretchable-cap approach by BioSemi leads, which is the more common method used in most conventional laboratory systems, yielded consistent electrode recording sites, but also requires effort to setup and clean. Interestingly, the dry electrode, pin-array approach of QUASAR also required effort to apply. The overall weight of the system was also a problem, leading to diminished comfort among most participants.
The three new, commercially oriented systems described here provide important advancements by pushing EEG technology towards wireless solutions and alternative approaches to establish connectivity between the electrode and the scalp through hair. Their wireless un-tethered operation makes them more applicable for use in every-day, less restrictive scenarios [6]. Thus, they are better suited for studies where the goal is to monitor neural activity within naturalistic scenarios, where whole body movements, normal social interaction, or minimal disruption to everyday behavior is necessary [6, 40].
However, this level of freedom and ease of application comes at the cost of substantially lower channel count, limiting their use to paradigms where high spatial resolution is not necessary. Additionally, the current, wireless EEG systems still fall short of allowing for comfortable, long-term wear for a variety of head sizes and hair types. While it is probable that dry, gel and preparation-free electrode designs will be needed for more widespread adoption in real-world settings, additional work must be completed to refine the current approaches for realistic applications. In particular, performance criteria regarding specific design features affecting usability should be established. Using these as a guidepost, future designs can then continue to improve on these advancements and enable ease of use in real-world environments while still providing the needed consistency in electrode recording location, synchronization with external events, comfort and overall wearability of the system for the participant.
Acknowledgements
We thank Mr Theodric (Theo) Feng, Dr Alfred Yu, Dr Vernon Lawhern, and Dr Troy Lau for their assistance in data collection, and Theo for taking pictures used in the paper. We would also like to thank the members of the Translational Neuroscience Branch at the US Army Research Laboratory for helpful discussion and insight over the course of this project.
Conflict of interest
The authors declare no conflicts of interest.