Abstract
To achieve a three-dimensional (3D) microenvironment for complex tissue regeneration is a great challenge when developing biomaterials as artificial extracellular matrix (ECM) with properties similar to that of native tissue. Polysaccharide-based hydrogel shows great potential as ECM in the regeneration of damaged tissues or reconstruction of organs, demonstrating properties similar to those of native ECM. Extrusion 3D printing of cell-free or cell-loaded hydrogel ink has led to a more sophisticated fabrication of the desired compositions and architectures for tissue engineering applications. The development of stable cell-free and cell-loaded hydrogel inks with optimal physicochemical properties and biocompatibility is also a major concern in direct-write extrusion-based 3D printing. In this study, carboxylated cellulose nanocrystals (cCNCs) were prepared using ammonium persulfate, where transmission electron microscopy, Fourier-transform infrared spectroscopy, and x-ray diffraction analyses confirmed their successful preparation. Further, the effect of cCNCs (–COOH) and/or xanthan gum (XG) (–COOH) was evaluated on the rheological behavior of the sodium alginate (SA) hydrogel matrix. The incorporation of cCNCs and XG manipulated the flow and shear-thinning behavior of the hydrogel inks, thereby improving the printing ability. The results showed good rheological properties, post-printing fidelity, and dynamic mechanical properties under compression of the developed hydrogel inks. Furthermore, good viability of the human skin fibroblast (CCD-986Sk) cells on bulk hydrogels (hydrogel inks) was observed, as demonstrated by both qualitative and quantitative cell analyses. The use of cCNCs and XG in SA hydrogel inks provides a primary insight for further improvement in designing 3D bioprintable hydrogel inks.
Export citation and abstract BibTeX RIS
1. Introduction
The achievement of a native three-dimensional (3D) microenvironment of tissue using artificial extracellular matrix (ECM) through cell attachment, proliferation, migration, and differentiation as well as the transport of biomolecules and removal of waste metabolites is a great challenge in tissue engineering. Hydrogels may provide promising 3D features of native ECM largely as structural support in terms of a highly hydrophilic 3D network having high water absorption capacity, elasticity, and diffusivity of small molecules. The fundamental cell–ECM interactions within the in vivo 3D microenvironment are highly dynamic in nature [1]. In addition, the control over pore size precision and anatomical design, including the distribution of cells and growth factors, are important factors to improve the efficacy of new tissue formation, which is difficult to achieve by conventional methods. To overcome these limitations, 3D printing has been used in tissue engineering, but the main challenge is to prepare printable biomaterials (i.e. hydrogel ink or bioink) [2, 3] to control the structure and functionalities of the biomaterials locally by aligning anisotropic particles in intended orientations to reach site-specific properties [4]. Among other biofabrication or 3D printing technologies, direct-write extrusion is a cost-effective, scalable, fast, and versatile technique [5]. This technique depends mainly on extrudable cell-free hydrogel inks or cell-loaded hydrogel inks with suitable rheological properties (i.e. viscosity, elasticity, and shear-thinning behavior) via a narrow nozzle using pneumatic or mechanical forces (screw-drive or piston) [6, 7]. Therefore, the development of new hydrogel inks or bioinks is gaining much attention due to the lack of suitable mechanical properties that are needed for precise printing and cell survival during the printing process. In recent times, the hydrogel inks and/or bioinks have been developed using several natural and synthetic polymers in the 3D printing process [7]. In this case, natural polymers (i.e. polysaccharides) may provide a better option due to their excellent cytocompatibility and biodegradability. However, they possess few mechanical properties that are needed to maintain their structural integrity (i.e. post-printing shape fidelity) as well as support physical stress within the in vivo 3D microenvironment [8, 9].
Polysaccharides such as sodium alginate (SA), xanthan gum (XG), and cellulose nanocrystals (CNCs) have attracted great attention as biomaterials in tissue engineering due to their native excellent biocompatibility and high hydrophilicity that can mimic natural ECM [10–13]. SA facilitates fast gelling behavior (with –COO− Na+) in the presence of divalent ions (e.g. Ca2+) and is a promising candidate for developing layer-by-layer SA-based hydrogel inks and/or bioinks using 3D printing [14–16]. In addition, the use of SA as a component in bioink is beneficial in accelerating proteoglycan synthesis [17]. However, SA itself exhibits a number of drawbacks such as uncontrolled and slow degradation kinetics under physiological conditions, but ionically crosslinked SA hydrogel shows limited long-term stability under physiological conditions due to the release of divalent ions into the surrounding media by exchanging with mono-valent cations [18, 19]. Therefore, SA alone possesses a lack of bioactivity and mechanical stability which limits its tissue engineering applications. In addition, XG has highly pseudoplastic behavior and apparent viscosity that significantly alters with time or shear rate [12, 20]. It possesses superior rheological properties (highly viscous at low shear forces and shear-thinning behavior at high shear forces) and thermal properties (against hydrolysis), but a lack of mechanical properties. XG also facilitates weak gel formation in the presence of divalent ions (e.g. Ca2+). Therefore, the shear-thinning and gelling behavior of XG might be more beneficial in extrusion-based 3D printing of bioinks in tissue engineering [12]. Further, nanocellulose (e.g. CNCs) also demonstrated potential efficacy in tissue engineering and antimicrobial biomaterials [21–23]. In 3D printing, the collapsing and shape fidelity of the biomaterials (especially polysaccharides) is a major problem for stable microstructure or bioconstruct [24] after the printing of hydrogel inks. Therefore, nanocellulose has been used for modifying rheological behavior (i.e. viscoelastic properties) of the hydrogel solution or paste to promote printability of the hydrogel biomaterial and post-printed shape fidelity [25]. CNCs, as anisotropic reinforcing particles, show a low density and high aspect ratio, excellent biocompatibility, high surface area (∼700 m2 g−1) and elasticity, high hydrophilicity, and provide excellent mechanical strength and stiffness to the composite biomaterials via self-assembly of CNCs in a unique hierarchical network [13, 26–28]. In addition, due to the large number of hydroxyl (–OH) groups on their surface, CNCs have been functionally modified for value-added biomaterials for various biomedical applications [21, 22, 29]. In our previous studies, we prepared CNCs (–OH) from microcrystalline cellulose (MCC) by using acid hydrolysis (sulfuric acid, H2SO4) for tissue engineering applications [10, 13, 27, 30]. Recently, ammonium persulfate (APS) has been used to prepare spherical [29] and uniform rod-like [31] CNCs with carboxylic (–COOH) groups on their surface [32]. APS is a strong oxidizer with low cost, high water solubility, and low toxicity for long-term use [33]. We prepared carboxylated CNCs (cCNCs) using APS and, in addition to their nanofiller efficiency, cCNCs provide extra crosslinking sites further in the hydrogel network to improve the mechanical properties as well as the shear-thinning behavior during extrusion (i.e. preferable orientation in the printing direction in 3D bioprinting) [25, 34].
In recent times, various hydrogel formulations with natural and/or synthetic polymers have been developed with and without nanocellulose for 3D bioprinting in tissue engineering applications. These include SA/collagen [35], SA/gelatin [36], SA/CNCs [37, 38], SA/gelatin/CNCs [34], SA/gelatin/bioactive glass/cellulose nanofibers [39], XG/carrageenan/starch [40], etc. Therefore, by taking advantage of cCNCs as a physical and chemical crosslinking agent and XG as an excellent gelling biomaterial with shear-thinning properties, new polysaccharide-based hydrogel inks or bioinks can be designed and developed for 3D bioprinting in tissue engineering. To our best knowledge, no study has reported taking the synergistic effects of XG and CNCs (especially cCNCs) in the SA matrix to design cell-free hydrogel inks or cell-loaded bioinks for 3D bioprinting in tissue engineering by investigating their structural, rheological, printability, mechanical, and cytocompatibility properties.
In this study, we prepared cCNCs by the APS method and incorporated them in SA/XG matrix to develop cell-free hydrogel inks for 3D bioprinting to print stable and biocompatible 3D hydrogel constructs. The physicochemical properties of the hydrogel inks were evaluated based on their structural, morphological, rheological (i.e. elastic and viscous modulus, shear-thinning property), and mechanical properties (strength and dynamic stiffness under compression). Furthermore, in vitro cytocompatibility analyses were performed with human skin fibroblast cells (qualitatively, using field emission scanning electron microscopy (FESEM) and Live/Dead assay, and quantitatively, using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay) in both non-printed (bulk) and printed hydrogels.
2. Materials and methods
2.1. Materials
SA (molecular weight: ∼50 kDa, M/G ratio: 1.67, viscosity: 100–300 cP for 2% solution at 25 °C), XG from Xanthomonas campestris (G1253, viscosity: 800–1200 cps), MCC, APS, and calcium chloride (CaCl2) were purchased form Sigma-Aldrich Co. Ltd (USA).
Dulbecco's modified Eagle's medium (DMEM), fetal bovine serum (FBS), penicillin G-streptomycin and trypsin-EDTA were provided by Gibco (Carlsbad, USA). Glutaraldehyde solution (50%), phosphate-buffered saline (PBS), MTT assay and the Live/Dead assay kit were purchased from Sigma-Aldrich (Yongin, Korea). Human skin fibroblast cells (CCD-986Sk) were obtained from the American Type Culture Collection (ATCC; Manassas, USA). All chemicals were used as received and de-ionized water (DIW) was used throughout the experiments. In this study, an Inkredible+ 3D printer (Cellink Co. Ltd, Sweden) was used to print hydrogel filament constructs and 3D printed structures.
2.2. Preparation of cCNCs
cCNCs were prepared by one-step oxidation of MCC using APS. In brief, 5 g MCC was added to 1 M APS 500 ml aqueous solution (pre-heated) under vigorous mechanical stirring for 16 h at 75 °C. Then, the suspension of CNCs was centrifuged and the obtained sample was washed with DIW at 10 000 rpm for 10 min until pH ∼4.0–5.0. This pH was further adjusted to 8 by adding 1 M sodium hydroxide (NaOH) and re-dispersed in 300 ml DIW by sonication for 20 min. The final suspension was stored in a refrigerator (at 4 °C) before further use. The yield of CNCs was measured as 45.6% based on gravimetric analysis [41].
2.3. Preparation of composite polysaccharide hydrogels
Aqueous suspensions of cCNCs were prepared with different amounts as 0.350 g, 0.175 g, and 0.0875 g in 25 ml DIW, respectively. Then, various amounts (0.0375 g, 0.0750 g, and 0.150 g) of XG with SA (fixed amount 0.63 g) were added in these aqueous suspensions of cCNCs at a percentage ratio of 55/6, 28/12, and 14/24 (CNCs/XG) in accordance with the amount of SA, respectively. All hydrogel suspensions were homogenously mixed under constant mechanical stirring at 60 °C for 5 h. We fixed the concentration of SA at 2.5 wt% and only varied the amount of CNCs (from 55 wt% to 14 wt%) and XG (from 6 wt% to 24 wt%) relative to the total amount of SA. For comparative analysis, cell-free hydrogel inks of SA with CNCs (55 wt%) or XG (24 wt%) were also prepared. Finally, all cell-free hydrogel inks were subjected to five freeze–thaw cycles (room temperature to −20 °C to room temperature). The formulations were designated as BPC55X6, BPC28X12, BPC14X24, BPC55, BPX24, BP, where BP, C, X denote SA, cCNCs, and XG, respectively, and are reported in table 1). In addition, schematic diagrams of the printing of hydrogel ink and the suggested reaction mechanism with the synergistic effect of the components in the hydrogel network are shown in figure 1. All hydrogel inks were stored in sterile conditions in a refrigerator at 4 °C [42].
Table 1. Formulations of cell-free hydrogel inks.
| Formulation | SA (g) | CNCs (g) | XG (g) | Water (g) |
|---|---|---|---|---|
| BP | 0.63 | 0 | 0 | 25 |
| BPX24 | 0.63 | 0 | 0.150 | 25 |
| BPC55 | 0.63 | 0.350 | 0 | 25 |
| BPC55X6 | 0.63 | 0.350 | 0.0375 | 25 |
| BPC28X12 | 0.63 | 0.175 | 0.0750 | 25 |
| BPC14X24 | 0.63 | 0.0875 | 0.150 | 25 |
CNCs = 1.4%.
Figure 1. Schematic diagrams of printing of BPCX hydrogel ink and suggested reaction mechanism with synergistic effect of the components.
Download figure:
Standard image High-resolution image2.4. Printing of cell-free hydrogel inks for 3D constructs
In order to show the potential printing ability of hydrogel inks, we chose a low concentration of BP solution (2.5 wt%) and investigated the blend with XG (gelling matrix) and cCNCs (nanofillers) to allow stable extrusion as compared to the high concentration of BP solution. Here, we speculated that a higher concentration would likely make the hydrogel inks too viscous, making them unable to extrude and even difficult to remove trapped bubbles. Further, this also facilitated non-continuous printing of the hydrogel inks and high pressure would be required for printing, where consequent shear forces may result in a lower viability of encapsulated cells, if used, during printing [36].
The three components of BP, XG, and cCNCs were blended to make multi-polysaccharide hydrogel formulations (see hydrogel inks in table 1) for printing a stable construct at room temperature. The prepared hydrogel inks were loaded into a clear syringe for printing the hydrogel constructs. The test was performed using a plastic cell culture petri dish (printing surface). Here, we analyzed the printability and stability while printing and after printing. We used the Inkredible+ bioprinter (Cellink, Sweden) to investigate the printability of hydrogel inks at 25 °C . For the extrusion, 25-gauge (25 G, inner diameter 410 μm) sterile standard conical bioprinting nozzles (Cellink, Sweden) were fixed to 3.0 ml plastic cartridges (2.0 ml hydrogel ink) for the printing. For constant extrusion, the required pneumatic pressure was set using the pressure control knob (PH1) and the distance from the needle to the printing surface was calibrated and optimized for consistent print line flow along with needle. For printing, a 5 × 5 grid (TissueModel_PCL.gcode; 10 × 10 × 1.2 mm, single PH, three layers, feed rate 60 mm min−1) was used. The parameters for printing were set based on the printability of the hydrogel inks. The printing temperature and linear speed of the head were set to 25 °C and 1.0 mm s−1, while the pressure was varied from 45 kPa to 95 kPa as per the continuous extrusion of the hydrogel inks. After printing, the printed constructs were subjected to crosslinking with 100 mM CaCl2 aqueous solution, which was poured on it and retained for 5 min. Then, we digitally imaged each one at a time to show the stability of the printed construct.
2.5. Characterization
2.5.1. Transmission electron microscopy (TEM)
The morphology of the cCNCs was analyzed using TEM (FEI Tecnai G2 F20) with 100–120 kV acceleration voltage. Before analysis, a dilute aqueous suspension of cCNCs was prepared by sonication and deposited on the copper grid surface (coated with a thin carbon film) followed by drying at room temperature under a lamp.
2.5.2. FESEM
The surface morphology of the 3D printed hydrogel constructs was analyzed using FESEM (S-4800, Hitachi Co. Ltd, Japan). Before analysis, the crosslinked printed constructs were freeze-dried (−80 °C), mounted on a stub, and coated with a fine layer of platinum at a low deposition rate.
2.5.3. Fourier-transform infrared (FTIR) spectroscopy
The chemical changes in MCC before and after APS treatment and crosslinked hybrid hydrogels were evaluated by using FTIR spectroscopy (Perkin Elmer). The degree of oxidation (DO) of the cCNCs was also measured from FTIR spectra using the following equation [43]:
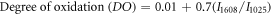
where I1608 is the intensity of the carboxylic (–COO−Na+) group and I1025 is the intensity of the highest absorbance peak from the structural IR spectra.
2.5.4. X-ray diffraction (XRD) analysis
The crystalline nature of the hybrid hydrogels was evaluated using XRD (Bruker AXS D8 Advance) at 40 kV and 30 mA with CuK radiation at a wavelength of 1.54 Å. The samples were scanned at diffraction angles in the range of 2θ = 10–70° with a scanning rate of 10 °C min−1 at room temperature. The crystallinity (%) was calculated by using following equation [44]:
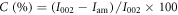
where I002 is the maximum intensity peak at 2θ = 22.5° and Iam is the minimum intensity between the planes (101) and (002).
2.5.5. Rheological analysis
Rheological analysis of hydrogel inks (before crosslinking with Ca2+ions and freeze–thaw cycles) was performed with a Rheometer Physica MCR 301 (Anton Paar GmbH, Graz, Austria). A parallel plate setting (PP25) was used for temperature (25 °C–50 °C), shear rate, and frequency-dependent analyses between 0.1–100 s−1. 1 mm distance was maintained between two parallel plates and the temperature of the rheometer lower plate was set to 25 °C.
2.5.6. Dynamic mechanical analysis (DMA)
The compressive strength of hydrogels was performed using dynamic mechanical analysis (DMA 800SDT) under unconfined compression with an 18 N load cell. Samples were initially placed under the pre-load force of 0.05 N and a rate of 3 N min−1 at room temperature (25 °C). For approximate analysis, bulk cylindrical shaped hydrogels were prepared by pouring hydrogel solutions in a 96-well plate as a mold, subjecting them to 5 freeze–thaw cycles, and then crosslinking with CaCl2 and washing to remove excess Ca2+ ions [45]. Finally, these hydrogels were cut using a sharp surgical blade to prepare 6 mm × 6 mm size sections without macropores. The size dimensions of the hydrogels were measured using digital callipers. These bulk hydrogels were used rather than 3D printed constructs in order to maintain consistency and uniformity in the measured data, excluding potential effects of the printing. The results are shown in stress–strain curves and dynamic stiffness values were evaluated at 10, 20, 30, and 40% of strains to analyze stiffness behavior at different compressive forces. Three independent tests were performed and the average values are reported [36].
2.5.7. Fidelity and angle on edges
For printed constructs, the area of meshes was calculated with ImageJ 1.46 software (NIH). The fidelity on mesh area (%) was calculated by equation (1) [46]:
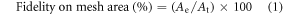
where At denotes the theoretical mesh area and Ae denotes the experimental mesh area. All mesh areas were averaged for an individual crosslinked printed construct (before freeze-drying) and compared with the theoretical mesh area (100%).
2.5.8. In vitro cytocompatibility
To evaluate the cytocompatibility of the bulk and printed hydrogel constructs with CCD-986Sk cells, we assessed the morphology (qualitatively by FESEM), cellular viability (qualitatively by Live/Dead assay), and cellular metabolic activity (quantitatively by MTT assay).
2.5.8.1. Morphological analysis
Cell adherence and spreading on hydrogel surfaces was analyzed by FESEM. In brief, hydrogels were sterilized with 70% ethanol followed by UV light prior to rinsing with PBS. Then, the sterilized hydrogels were soaked in culture medium (DMEM, 10% FBS, and 1% penicillin G-streptomycin) for 4 h and seeded with human skin fibroblast cells (CCD-986Sk, 1.0 × 105 cells ml−1). Then, cell-seeded hydrogels were incubated for 1, 7, and 14 days at 37 °C with 5% CO2 (humidified atmosphere). After the desired time periods, the culture medium was removed and samples were washed with PBS and respectively fixed with 2.5% glutaraldehyde for 15 min. The hydrogel samples were finally dehydrated and freeze-dried for morphological analysis.
2.5.8.2. Cell viability and proliferation
2.5.8.2.1. Live/Dead assay
A Live/Dead assay kit was used for cell viability analysis after 1, 7, and 14 days of cell culture. In brief, 20 μl of the supplied 2 mM ethidium bromide (EthD-1) stock solution was diluted with 10 ml of sterilized tissue culture-grade D-PBS and then subjected to vortexing to ensure thorough mixing of the final solution (4 μM EthD-1 solution). Further, 5 μl of supplied 4 mM calcein-acetoxymethyl ester (calcein-AM) stock solution was transferred to this 10 ml EthD-1 solution and mixed thoroughly by vortexing. CCD-986Sk cell-cultured BP (i.e. only SA) and other BP-based 3D bulk hydrogels including printed BPC55X6 hydrogel construct were washed with PBS and stained with the resulting solution (100–150 μl) for 25 min at 37 °C in the dark. Finally, labeled cells were examined using a fluorescence microscope and imaged by attached digital camera (IX81, Olympus, Tokyo). The number of live cells was counted under the microscope from different areas of each hydrogel sample. Finally, the cell viability was calculated based on the percentage (%) of live cells to total number of cells.
2.5.8.2.2. MTT assay
Metabolic activity of the cells on hydrogel was analyzed by staining with MTT assay. CCD-986Sk cells (human skin fibroblast cell line) were grown in culture medium (DMEM, 10% FBS, and 1% penicillin G-streptomycin) at 37 °C with 5% CO2 (humidified atmosphere). CCD-986Sk cells (1.0 × 105 cells ml−1) were seeded on hydrogel surfaces on a 24-well plate for 1, 7, and 14 days, respectively. In brief, MTT solution (200 μl from 5 mg ml−1) was added to each well and incubated at 37 °C for 4 h. The supernatant was aspirated and produced MTT-formazan crystals which were dissolved in DMSO solvent. The absorbance was analyzed by microplate reader (Bio-T Instruments, Inc., USA) at a wavelength of 490 nm.
2.5.9. Statistical analysis
All experiments were performed in triplicate. The results are given as the mean value ± standard deviation (SD) of these three independent tests. Multiple comparisons were carried out using one-way analysis of variance followed by the Duncan test for post hoc analysis using SPSS16 software. Statistically significant values were considered as p values <0.05.
3. Results and discussion
3.1. Morphological and structural analysis of cCNCs
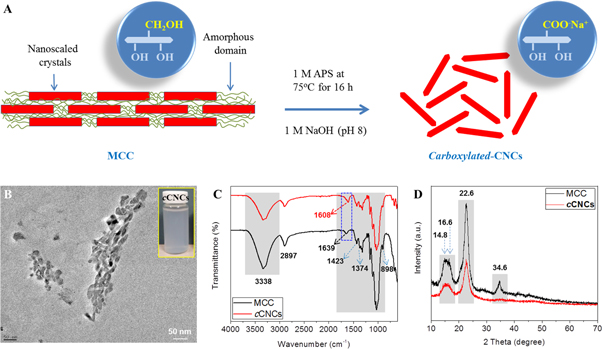
Figure 2(A) shows the schematic process of the one-step preparation of cCNCs from MCC using APS as a mild oxidizing agent. The morphology of the prepared cCNCs was analyzed by TEM (see figure 2(B)). TEM images showed mixed spindle-like and sphere-like shapes with diameter in the range of 15–30 nm and the length in the range of 30–120 nm (as measured by ImageJ 1.46 software). Further, the structural changes in the preparation of cCNCs from MCC by APS chemical treatment were analyzed by FTIR and XRD analysis (as shown in figures 2(C) and (D)). In figure 2(C), MCC shows the characteristic peaks of cellulose at around 3338 cm−1 (O–H stretching), 2898 cm−1 (C–H stretching), 1425 cm−1 (O–C–H and H–C–H deformation), 1372 cm−1 (C–H vibrations), and 898 cm−1 (C–H vibrations) [29, 47]. However, no significant changes were observed before and after APS treatment with MCC. In the case of cCNCs, a peak at around 1608 cm−1 was observed due to the incorporation of carboxylic (–COOH) groups in the ionized form (–COO−Na+) after the neutralization of cCNCs with NaOH) [31, 48, 49]. The main chemical structure of MCC was not affected by APS treatment and only the peak position of –COOH groups (in cCNCs) was affected significantly by the neutralization with NaOH (surface deprotonating of –COOH groups at around 1728 cm−1 due to C=O stretching) [50]. The DO of cCNCs was calculated at pH > 7 using the conductometric titration method as described elsewhere [50]. The DO value was 0.17 for cCNCs after the APS treatment [29, 51]. For confirmation, the DO of cCNCs was also calculated using the intensity of FTIR peaks at around 1608 cm−1 and 1025 cm−1 by the equation described in section 2.5.3 (FTIR analysis). The DO was calculated as 0.0975, which is similar to the value found using the chemical method.
Figure 2. (A) Schematic diagram of the one-step preparation of cCNCs from MCC by using APS. (B) TEM image of cCNCs, (C) FTIR spectra, and (D) XRD patterns of MCC and cCNCs, respectively.
Download figure:
Standard image High-resolution imageIn support of the FTIR analysis, XRD analysis was performed to investigate the crystalline changes after the introduction of –COOH (APS treatment). In figure 2(D), MCC shows characteristic peaks at around 2θ = 15.1°, 16.5°, 22.5°, and 34.5° (040) which are assigned to the characteristic peaks of the native cellulose I structure and after APS treatment, cCNCs also showed similar characteristic peaks. However, the peak at around 34.5° was reduced. The crystallinity (%) of both MCC and cCNCs was observed to be almost similar (∼76.1% for MCC and ∼76.8% for cCNCs) [41].
3.2. Rheological analysis of hydrogel inks
To perform 3D printing of cell-free or cell-loaded hydrogel inks with post-printing fidelity it is a major challenge to keep other desirable properties of the native 3D microenvironment in tissue regeneration. Therefore, we prepared new compositions of cell-free hydrogel inks and characterized their rheological behaviors before 3D printing. Importantly, we analyzed how the rheological and mechanical behaviors can be tailored by the change in the composition, including their enhanced crosslinking reaction mechanism for printing ability or post-printing fidelity and geometric accuracy. For this development of hydrogel ink or bioink, the designed hydrogels should provide sufficient viscosity for dispensing (free-standing gel filament), mechanical strength, and stiffness to maintain post-printing shape fidelity [45].
To evaluate the printability of BP and BP with XG and/or cCNCs, the rheological properties (elastic and viscous behavior) of hydrogel inks were measured as function of frequency, shear rate, and temperature (figures 3(A)–(D)).
Figure 3. Frequency- and temperature-dependent rheological analysis: (A) and (C) storage modulus and loss modulus, and (B) and (D) complex viscosity, respectively.
Download figure:
Standard image High-resolution image3.2.1. Frequency- and temperature-dependent rheological analyses
The synergistic effect of cCNCs and XG on the rheological properties of BP-based hydrogel inks was investigated. Here, a rotational rheometer was used to investigate the rheological properties of 3D printable hydrogels. Figure 3(A) shows the frequency-dependent storage modulus (G') and loss modulus (G''), whereas figure 3(B) shows the complex viscosity analysis.
In figure 3(A), for all hydrogels, the G' values are higher than the G'' values in the frequency range of 1–100 Hz at 25 °C. However, the combined effect of cCNCs and XG in BPCX hydrogel inks showed lower G' values compared to BPC hydrogel which further lowered with decreased and increased of amount of CNCs and XG, respectively. Due to the five additional freeze–thaw cycles, BP hydrogel ink showed good 'gel-like' behavior over this frequency range and significantly increased at higher frequency values, while the incorporation of XG (BPX24 hydrogel ink) increased G' values at lower frequency and significantly lowered them at higher frequency. Further, the incorporation of cCNCs (BPC55 hydrogel ink) significantly increased the G' values over the entire frequency range of 1–100 Hz, compared to both BP and BPX24 hydrogels. This is possibly due to the self-assembled liquid crystalline behavior of cCNCs [52]. Further, BPC55X6, BPC28X12, and BPC14X24 hydrogel inks showed a similar trend at lower frequency, but BPC55X6 hybrid hydrogel showed somewhat lower G' values at higher frequency compared to BPC14X24 hydrogel ink, whereas BPC28X12 hydrogel ink exhibited much lower G' values at higher frequency compared to both BPC55X6 and BPC14X24 hydrogel inks. Possibly, this is due to the complex synergistic effect of both cCNCs and XG in the BP hydrogel network and contributed to the shear-thinning of the 3D hydrogel network. In addition, figure 3(B) showed a similar pattern of increased complex viscosity (Pa.s) with increased amount of XG and/or CNCs at lower frequency value. However, the complex viscosity (Pa.s) was observed to be reduced and the slope of all formulations (BP and BPCX hydrogels) became steeper as the frequency increased. It can be concluded that both cCNCs and XG significantly increased the shear-thinning behavior of the hydrogel ink [38].
Figures 3(C) and (D) show the temperature-dependent rheological analysis of BP and BPCX hydrogel inks. BP hydrogel showed good thermal stability in the 25 °C–50 °C range of temperature. The temperature-dependent data revealed that the transition temperature increased or decreased with the incorporation of cCNCs and XG. The carboxylic (–COOH) groups in cCNCs and XG decreased the gelation temperature of BP matrix due to the dehydration of SA [53]. Furthermore, with the increase in temperature over the gelation temperature to 50 °C, the mechanical properties of hybrid hydrogels increased as can be observed from the increased G' values [54]. The sol-gel transition (G' becoming higher than G'') was observed in all hydrogel inks with increased temperature due to the decreased polymer–solvent interactions, while improving strong hydrophobic interactions [55, 56]. Although BP hydrogel showed higher G' values than G'' values, it is not enough for gel formation with good elastic properties for stable printing of hydrogel ink. This gel-like characteristic with good elastic nature was improved by incorporating XG and/or cCNCs in BP matrix. Further, the hydrophilic nature of cCNCs and XG facilitates high polymer–solvent interactions, but no significant improvement was observed in gel formation. However, cCNCs and XG can effectively be utilized for developing 3D printable cell-free hydrogel inks and cell-loaded hydrogel inks with tunable properties that may have various tissue engineering applications [57].
3.2.2. Evaluation of shear-thinning behavior
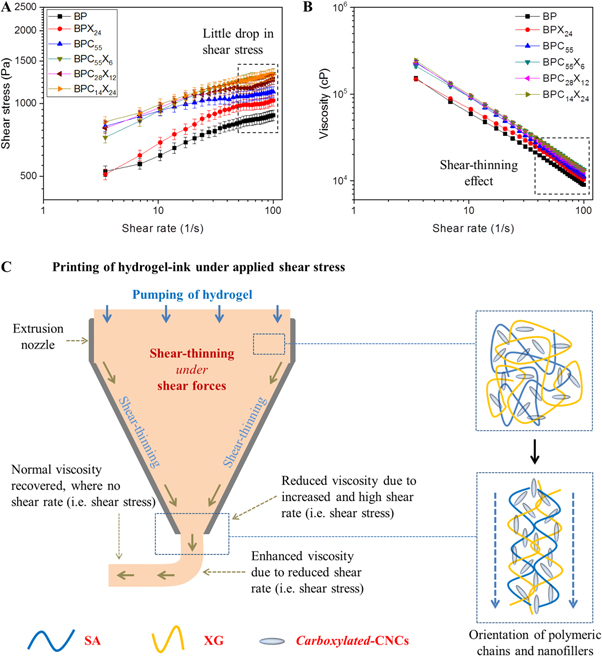
To analyze this behavior, shear stress analysis for shear-thinning behavior was measured as a function of shear rate (see figures 4(A) and (B)). At higher shear rate, BP hydrogel inks bear high shear stress in dispensing the hydrogel filament through the extruder (see figure 4(A)), whereas shear stress slightly decreased when XG was incorporated in the BP matrix as in BPX24 hydrogel ink. Similar behavior was also observed in BPC55 and BPC28X12 hydrogels, while this effect was not so significant in the case of BPC55X6 and BPC14X24 hydrogel inks due to the synergistic behavior via restricted alignment (entanglement through extensive physical crosslinking) of SA/XG chains in BPC14X24 hydrogel and low shear-thinning effect of cCNCs in BPC55X6 hydrogel [4]. Further, figure 4(B) shows a similar trend to figure 3(B). The viscosity (cP) of the hydrogel inks reduced and the slope of all formulations of hydrogel inks, especially hydrogel inks with cCNCs and/or XG, became steeper as the shear rate (1/s) increased.
Figure 4. (A) Applied shear stress and (B) viscosity of the hydrogel inks with varying shear rate in the range of 1–100 1/s. (C) Schematic representation of the behavior of hydrogel inks under applied shear stress via conical extrusion nozzle.
Download figure:
Standard image High-resolution imageThis shear-thinning behavior can be understood using the suggested schematic mechanism of the behavior of hydrogel inks via the extrusion nozzle tip model, as shown in figure 4(C). Before entering into the conical section, polymer chains and nanofillers are randomly arranged in the hydrogel inks, whereas in the conical section, hydrogel inks bear more shear forces. In this case, the formed hydrogel network is broken up under shear forces and the polymer chains as well as nanofillers aligned to result in reduced viscosity. Hydrogel inks exhibit increased shear-thinning behavior (i.e. reduction in viscosity) from the upper section to the lower section of the nozzle tip. When shear stress was removed (outside the extruder tip), this aligned hydrogel network of polymeric chains and nanofillers again recovered to normal form (i.e. initial viscosity) due to thixotropic behavior and instantly exhibited a stable printed filament line at the printing surface. This is possibly due to the shear-thinning property of XG as well as cCNCs of high aspect ratio that aligned parallel when extruded out and reversed to normal viscosity via anisotropic behavior of cCNCs or percolation effect (figure 4(C)).
3.3. Hydrogel formation and printing process
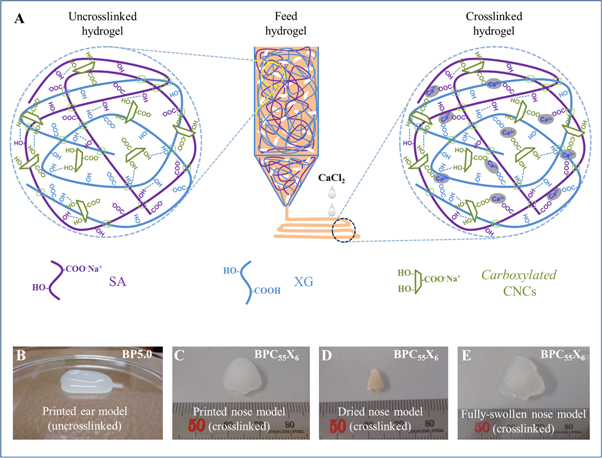
The structural changes in chemical functionalities after hydrogel formation are shown in figures S1(A) and (B) (available online at stacks.iop.org/BF/12/025029/mmedia). Further, figure S2(A) shows a digital image of the 3D bioprinter (Cellink Inkredible+ model, Sweden) used for printing BP hydrogel ink in a three-layer construct. The initial printing of the hydrogel construct (i.e. BP) can be seen in figure S2(B). To understand the printing process and chemical crosslinking, a schematic of the dispensing of cell-free BPCX hydrogel ink is given in figure 5(A). In addition, a suggestive reaction mechanism is provided before and after crosslinking with CaCl2. Before crosslinking, there is only extensive hydrogen bonding, while after crosslinking both hydrogen bonding and ionic crosslinking are facilitated, which make it a physically stable hydrogel network with dynamic mechanism under external shear forces. The viscosity of the hydrogel inks was low enough to avoid high printing pressures to allow extrusion, and high enough to print stable filament lines or printed constructs. Figure 5(B) shows the printed ear model to analyze the stability of the construct using BP (5.0 wt%) hydrogel ink. However, BP (2.5 wt%) hydrogel ink was also used for the printing of this ear model, but this printed construct was not as stable (image is not shown here) and collapsed very quickly, diminishing the ear structure before crosslinking. In addition, to investigate the printing stability in 3D constructs, a human nose model (Human nose_scale 50) was also printed using BPC55X6 hydrogel ink with 128 kPa pneumatic pressure (fast printing) (see figure 5(C)) for the printed nose model, figure 5(D) for oven-dried, and figure 5(E) for the fully swollen nose model in PBS). This swelling of the dried nose model demonstrates that the printed nose model construct did not lose its architecture or anatomy largely. In addition, we have also printed some hydrogel formations by free-hand without using the Cellink Incredible+ bioprinter (as shown in figure S3).
Figure 5. (A) Schematic diagram of the dispensing of BPCX cell-free hydrogel inks followed by the mechanism of hydrogel construct crosslinking with or without CaCl2. (B) Digital image of printed BP (5.0 wt%) ear model (uncrosslinked, only hydrogel bonding), (C) printed nose model, (D) oven-dried, and (E) fully swollen printed nose model in PBS (BPC55X6 crosslinked with CaCl2).
Download figure:
Standard image High-resolution image3.4. Printing stability and post-printing fidelity of hydrogel inks
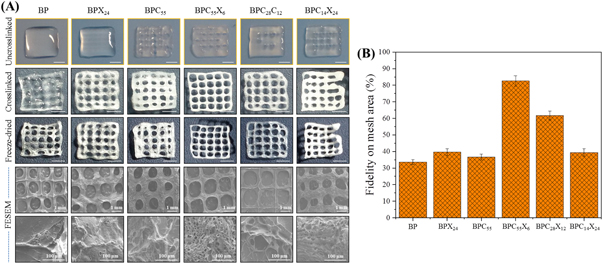
Figure 6(A) shows the digital and FESEM images of the post-printing stability of the various hydrogel inks (with or without crosslinking). After 30 min, the uncrosslinked printed construct of BP converted to a bulk hydrogel-type construct and no clear filament lines were observed. Further, BPX24 also converted to a bulk hydrogel-type construct, but filament lines could be observed inside the printed construct. However, the incorporation of cCNCs showed a stable printed construct (see BPC55) showing almost clear printed filament lines. Further, the incorporation of cCNCs and XG together as in BPC55X6 hydrogel ink also synergistically showed good printing stability with almost clear printing filament lines. Additionally, BPC28X12 and BPC14X24 also showed similar behavior. However, in all cases, the filament lines somewhat mingled with each other due to the flow of hydrogel inks. In addition, the crosslinked printed constructs exhibited good post-printing stability and all formulation types showed continuous and clear printing of hydrogel constructs, but the filament line width was observed to be higher compared to the extrusion nozzle tip inner diameter. However, the printed constructs were influenced and dwindled a little (∼10%–15% reduction in their printed size dimensions) by chemical crosslinking as compared to the uncrosslinked printed constructs. Here, an extensive hydrogen-bonds build up in hybrid hydrogels might strengthen before and after printing of hydrogels, and formed stable 3D printing of hydrogel ink. Further, the digital images of freeze-dried printed constructs show the morphology of BP and BP with cCNCs and/or XG at lower magnification (scale bar: 1 mm). However, the crosslinked printed constructs did not show significant changes in the overall printed structure after freeze-drying. The fidelity on mesh area (%) of the printed constructs was also measured, as shown in figure 6(B). BP exhibits a much smaller mesh area (∼33.6%) compared to the theoretical mesh area (100%), where it was hard to keep clear and stable filament lines in the printed construct. Further, the incorporation of XG in SA matrix (i.e. BPX24) increased in the mesh area (∼39.6%) with stable filament lines, whereas the incorporation of cCNCs in SA matrix (i.e. BPC55) showed a somewhat lower mesh area (∼36.6%) than that of BPX24. When both cCNCs and XG were added to the SA matrix (i.e. BPC55X6), clear printed filament lines with a higher mesh area (∼82.6%) was observed compared to BP, BPX24, and BPC55 printed constructs, but lower than that of the theoretical mesh area of the model construct. However, the mesh area was decreased to ∼61.8% and ∼39.4% for BPC28X12 and BPC14X24, respectively. This is due to the gelling property of XG and high water absorption ability which forms thick filament lines after the extrusion of hydrogel ink. Therefore, the performance of BPC55X6 hydrogel ink was good and the mesh area was closer to the theoretical mesh area (100%) compared to other hydrogel inks. In addition, BPC55X6 and BPC28X12 hydrogel inks showed the lowest deviation from the theoretical edge angle (0–90°), while other compositions showed almost round mesh areas (figure S4).
Figure 6. (A) Post-printing fidelity or stability of non-crosslinked printed constructs using different hydrogel inks after 30 min (scale bar: ∼3.5 mm), instant crosslinking of printed constructs (scale bar: ∼2.5 mm), respective dried crosslinked printed constructs (scale bar: ∼2.3 mm), and FESEM images of dried printed constructs at low magnification (scale bar: 1 mm) and high magnification (scale bar: 100 μm), respectively. (B) Fidelity on mesh area (%) of the printed constructs.
Download figure:
Standard image High-resolution imageThese FESEM images showed a homogenous microstructure with a consistent macroporous structure and maintained their integrity of printed filaments, but almost collapsed filament lines were observed. This is possibly due to freeze-drying, where finely printed constructs collapsed and very thin printed filament lines did not hold the pressure balance (i.e. applied vacuum pressure) during the freeze-drying process. However, a porous microstructure of the printed filaments can be observed even after the collapsing of printed filament lines, especially in BPC55, BPC55X6, BPC28X12, and BPC14X24 printed constructs at high magnification.
3.5. DMA
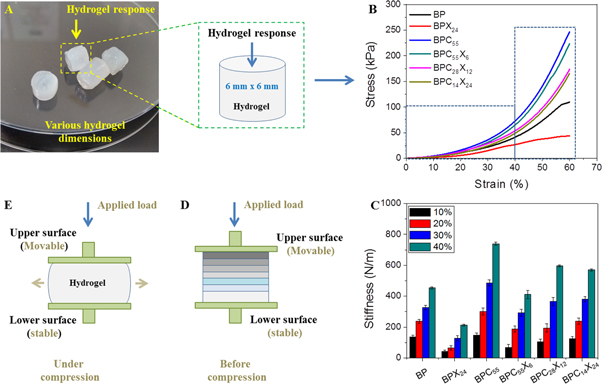
The dynamic behavior of the biomaterial under a 3D microenvironment (i.e. in vivo shear forces) is an important factor in the regeneration of tissue via cell attachment, proliferation, migration, and differentiation. In the bulk hydrogels, this dynamic behavior is varied from the outer part to inner part. Therefore, we analyzed the mechanical behavior of fully swollen bulk hydrogels under compression. Figure 7(A) shows the digital images of only BPC55X6 hydrogel of various sizes. For hydrogel response, the compressive mechanical properties of the crosslinked hydrogels were evaluated by DMA under unconfined compression (see figure 7(B)), where the composition and crosslinking effect might have a synergistic effect on the strength and stiffness of hydrogels. In figure 7(B), BP hydrogel shows a good stability (109.4 ± 3.5 kPa) of the hydrogel network due to extensive physical crosslinking (five freeze–thaw cycles) and chemical crosslinking (ionic interactions). However, the incorporation of XG in BP matrix (BPX24) decreased the compressive strength (42.02 ± 3.1 kPa) and this might be due to the weak gel behavior of XG and the broken intra- and intra-molecular hydrogen bonding of the BP hydrogel network under unconfined compression, making overall a weak hydrogel network compared to BP hydrogel. Further, the incorporation of cCNCs in the BP matrix (BPC55) increased the compressive strength (246.9 ± 5.8 kPa) tremendously compared to BP and BPX24 hydrogels, due to the high elastic modulus and effective load transfer ability of cCNCs [58].
Figure 7. (A) Digital image of bulk hydrogels, (B) stress–strain curves, and (C) dynamic stiffness values of hydrogel inks. A schematic model of the behavior of crosslinked hydrogel before (D) and after (E) unconfined compression.
Download figure:
Standard image High-resolution imageIn addition, the incorporation of both cCNCs (high) and XG (low) content in the BP matrix (BPC55X6) increased the compressive strength (223.2 ± 2.6 kPa), but less than that of BPC55 hydrogels, and further, this strength value decreased as the content of cCNCs decreased and the content of XG increased in BPC28X12 (173.4 ± 3.9 kPa) and BPC14X24 (165.5 ± 6.2 kPa) hydrogels, respectively.
Dynamic stiffness values were also measured and showed complex behavior during the compression stress (see figure 7(C)). Therefore, dynamic stiffness values were measured at different strains (10, 20, 30, and 40%), as reported in table 2. Dynamic stiffness values followed a similar trend of compressive strength up to BPC55 hydrogel, but decreased significantly in case of BPC55X6 and further gradually increased for BPC28X12 and BPC14X24 hydrogels, respectively, at 10% and 20% of strains. In BPCX hydrogels, we speculate that the initially negligible load transfer of cCNCs (that were far apart) might be facilitated in hydrogel, and when more stress was applied, they came together to participate in the load transfer mechanism (behaving as a stiffer hydrogel network). Further, XG chains provide more entanglement of polymeric chains (due to freeze–thaw cycles) and fitness with cCNCs, which facilitate slightly more stiffness to the overall hydrogel network. The hydrogel network behaves differently under applied force during low and high unconfined compression (as can be seen in figures 7(D) and (E)). The synergistic effect of XG and cCNCs controls the rheological properties of the hydrogel inks during printing, and promisingly in an in vivo 3D microenvironment. The actual moduli values of the produced hydrogels are in 100 s of kPa which may be quite stiff for musculoskeletal tissues in vivo. However, the parameters are optimized for the print fidelity and some fine-tuning might be required to achieve physiologically relevant rigidity of the hydrogels. Here, we speculate that the dynamic stiffness values may have a positive impact on the initial cell adherence and growth, and further during tissue formation (in vivo dynamic 3D microenvironment).
Table 2. Compressive properties of hydrogels.
| Dynamic stiffness (N m−1) | ||||||
|---|---|---|---|---|---|---|
| Compressive strength (kPa) | Strain (%) | |||||
| Formulations | At 60% strain | Failure strain (%) | 10 | 20 | 30 | 40 |
| BP | 109.4 ± 3.5 | ∼57 | 135.6 ± 10.2 | 238.1 ± 13.4 | 326.2 ± 15.5 | 454.3 ± 7.3 |
| BPX24 | 42.02 ± 3.1 | ∼50 | 41.39 ± 14.1 | 64.25 ± 14.7 | 129.1 ± 17.3 | 213.4 ± 6.1 |
| BPC55 | 246.9 ± 5.8 | ∼67 | 147.6 ± 19.5 | 301.1 ± 24.0 | 484.7 ± 20.6 | 739.9 ± 9.4 |
| BPC55X6 | 223.2 ± 2.6 | ∼65 | 69.35 ± 18.0 | 187.2 ± 20.3 | 366.1 ± 24.4 | 596.7 ± 26.8 |
| BPC28X12 | 173.4 ± 3.9 | ∼69 | 105.2 ± 15.6 | 194.2 ± 28.2 | 292.6 ± 27.3 | 411.5 ± 5.3 |
| BPC14X24 | 165.5 ± 6.2 | ∼68 | 125.3 ± 10.3 | 238 ± 20.3 | 381.5 ± 17.4 | 570.2 ± 7.6 |
3.6. Cytocompatibility
3.6.1. Cell morphology
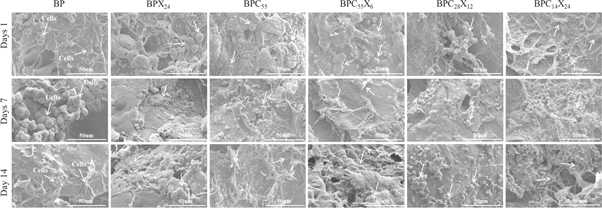
Morphological analysis of cells on hydrogel or scaffold is an important factor in analyzing biochemical functions involved in tissue formation. These biochemical functions in a native 3D microenvironment are dependent on several factors (e.g. density, hydrophilicity, stiffness, elastic modulus, and degradation of the hydrogel) [59, 60]. FESEM images (as shown in figure 8) showed cells spreading with round morphology to hydrogel surfaces after 1 day of culture and good spreading was observed. In general, BP (i.e. SA) hydrogel is biodegradable and biocompatible, but is not able to interact specifically with mammalian cells [61, 62]. However, the cells on BP hydrogel might have little cell adherence on the hydrogel surface due to scratches or irregular surface characteristics, or be physically adsorbed on hydrogel surface followed by the aggregation of cells (by forming clusters of cells), due to more cell–cell interactions than cell–matrix interactions [63]. Further, large numbers of cells were tightly adhered to the hydrogel surfaces and had migrated through pores after 7 days of culture. Additionally, highly populated cell adherence, spreading, and aggregation to make stratified cell layers by covering almost all hydrogel surfaces was observed after 14 days of cell culture.
Figure 8. FESEM images of cell-cultured hydrogels showing cell spreading and growth (scale bar = 50 μm) for (the presence of cells is indicated by the arrow).
Download figure:
Standard image High-resolution image3.6.2. Cell viability and proliferation
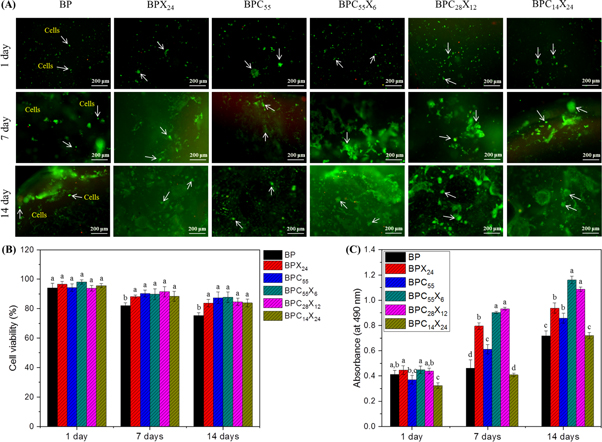
Cell viability and proliferation analysis (Live/Dead assay and MTT assay) also supported the cell morphological analysis (FESEM). The Live/Dead assay as a qualitative analysis was performed on 3D bulk hydrogels to visualize the distribution of live (green color) and dead (red color) cells after 1, 7, and 14 days of cell culture (see figure 9). In this analysis, calcein-AM stains live cells a green color by facilitating the reaction of intracellular esterase, whereas ethidum homodimer 1 (EthD-1) stains dead cells in red color by binding to the DNA of dead membrane compromised cells. For this, CCD-986Sk cells (1 × 105 cells) were cultured in bulk hydrogels for different time periods of 1, 7, and 14 days. As can be seen in figure 9(A), Live/Dead assay showed a distribution of viable cells homogenously in all hydrogel types and dead cells were hardly observed.
Figure 9. (A) Qualitative analysis (Live/Dead assay) of 3D BP and BP-based bulk hydrogels showing cell viability (representative florescence images) by staining with calcein-AM (live) and ethidum homodimer (dead) for 1, 7, and 14 days of incubation, respectively. Live and dead cells were stained green and red (scale bar = 200 μm). (B) Quantitative analysis for cell viability (%) and (C) MTT assay showing metabolic activity of CCD-986Sk cells in bulk hydrogels at 1, 7, and 14 days of incubation. Each value in the bar graph denotes the mean of three independent analyses ± SD. Values with different superscripts significantly differ from each other (p < 0.05).
Download figure:
Standard image High-resolution imageThe number of live cells was observed to be much higher than that of dead cells after 14 days of cell culture and very few dead cells or negligible death of cells were observed (see figure 9(B)). It was observed that cellular activity remained stable and showed no cytotoxicity on metabolic activity of the CCD-986Sk cells in hydrogels. Further, as reported in literature, viable cells can have the ability to remodel the hydrogel network and deposit their own ECM [64, 65]. Moreover, hydrogel surfaces were well adhered and covered with live (green) proliferating cells at higher density (see figure 9) and reflected the behavior as described by FESEM.
Here, we speculate that the density in the hydrogel network was increased due to ionic crosslinking and hydrogen bonding, while providing proper living space for CCD-986Sk cells and efficient transport of nutrients to the cells. The incorporation of cCNCs and/or XG in BPCX hydrogels is assumed to provide the required stiffness for cell adherence and growth (stiff substrate-based cell mechanics can be seen in figures S5(A) and shear-thinning effect in dynamic 3D microenvironment for cell viability compared to only BP hydrogel [60]. For comparison, a printed hydrogel construct (e.g. BPC55X6) was also used for Live/Dead assay after 14 days of cell culture showing stable cell viability with no cytotoxicity (see figures S5(B)–(D)).
Cell viability in hydrogels was further examined by MTT assay (see figure 9(C)). Compared to the 2D culture plate, hydrogels (3D) provide a larger space and higher surface area for cell proliferation and viability, and an extended proliferative activity (i.e. ability to survive) for a longer cell culture time period. After 1 day of incubation, BP showed good cell viability and further increased with the increasing time duration (from 7 days to 14 days). Overall, cells were observed to be viable and metabolically more active in all hydrogels and no significant difference was observed after 1 day of incubation. However, BPC55X6 hydrogel shows more significant cell viability apart from other hydrogel compositions for 1, 7, and 14 days of incubation. This is possibly due to the increased hydrogel stiffness which has a significant impact on cell fate [30].
3.7. Limitations
In this study, the extrusion behavior and printing stability of hydrogel inks via a single gauge (25 G) was investigated by the incorporation of XG and/or cCNCs. Here, we only evaluated the synergistic effect of XG and cCNCs on rheological behavior, printing ability and shape fidelity, mechanical properties of bulk hydrogels, and their in vitro cell cytocompatibility behaviour (adherence and viability). Furthermore, these biomaterials need more combinations for finding the optimized formulation for post-printing stability and fidelity, even without a crosslinker. For better understanding, the use of different gauge sizes (e.g. each blend with different gauge needles) may give a better insight into the hydrogel inks. The optimization of pneumatic pressure (i.e. shear stress) and increased concentration of the SA matrix by involving a high content of XG and/or cCNCs should be explored. Future studies may involve the effect of concentration, crosslinking agents (e.g. types, time), printing with pore size, compression testing with printed constructs (not bulk hydrogels), and in vitro degradation kinetics (in PBS or DMEM) of the printed constructs. Additionally, cell-loaded hydrogel inks (i.e. bioinks) must be analyzed for realistic behavior of the cells during printing. In this case, a cell survival study via the printing process for short-term and long-term behavior of the cells should be performed. These parameters may help in better understanding the printability and cell viability in bioinks. Therefore, further testing is needed to evaluate the proper printing of natural polymer-based hydrogel inks and bioinks [36, 66], by taking the benefits from our findings.
4. Conclusion
In this study, we developed multicomponent cell-free hydrogel inks composed of SA, XG, and cCNCs and analyzed post-printing fidelity using 3D printing for tissue engineering applications. Here, both XG and cCNCs provided a shear-thinning property to the hydrogel inks as well as extensive crosslinking sites (–COO− and –OH groups) in developing BP-based hydrogel networks with enhanced printability. In addition, cCNCs provide nanoscaled mechanical properties (strength and dynamic stiffness) to the BPCX hydrogel inks for cell adherence, proliferation, migration, and differentiation. Bulk hydrogels were able to provide a suitable 3D microenvironment for cells to adhere, proliferate, and migrate within the hydrogel network. Among all prepared hydrogel inks, BPC55X6 cell-free hydrogel inks demonstrated good printing stability and shape fidelity of printed filaments with good resolution and improved mechanical properties and cell viability. This research study may potentially provide an insight to aid understanding of the rheological properties of materials in designing and developing cell-free hydrogel inks or cell-loaded hydrogel inks for tissue engineering applications.
Acknowledgments
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (Grant No. 2017R1D1A3B03036276).