Abstract
Bacterial cellulose (BC) has attracted considerable scientific interest and can be modified, making it more widely useful in composites with guest nanoparticles. In this study, silica nanoparticles obtained from rice husks were used to prepare BC-silica composite aerogels (CAs) via a sol-gel method. Various amount of silica nanoparticles (3, 6, 9 and 12% w/v) dissolved in 2.5 M NaOH were used as a precursor for inclusion into BC. Subsequently, it was employed to form a SiO2 gel skeleton in a BC matrix by adding 2 M H2SO4, as a catalyst. Increasing levels of silica nanoparticles led progressively lower transmittance values of BC-silica CAs. SEM images revealed a surface morphology of spheroid particles with little agglomeration. The XRD diffraction peaks were gradually covered by a broad peak of silica as increasing silica content. Similarly, FTIR spectroscopy results also indicate the presence of silica in proportion to its content. Furthermore, addition of silica nanoparticles improved the thermal properties using TGA analysis, shifting the decomposition temperature of BC up to 550 °C and retaining of BC weight at least 60% with the BC sample with 3% of silica. This unique characteristic implies that silica had a stabilizing effect on polymeric cellulose. These results demonstrate an economical and environmentally friendly preparation of BC-silica CAs that can benefit material applications.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
In recent times, nanocomposites that contain various functional nanomaterials have attracted a great deal of attention in academic research due to the requirement for materials with improved physical and chemical properties. For example, inorganic and organic hybrid materials, especially nanostructured inorganic materials in polymeric matrices have attracted a great deal of attention due to the promise of combining the thermal and mechanical properties of inorganic compounds and polymers, respectively [1]. Consequently, polymer matrices with nanostructured inorganic composites show good potential in various electronics, photonics, catalysis, biomedical and environmental applications [2–7]. Cellulose, one of the most exploited renewable raw materials, is Earth's most abundant biopolymer and a major component of wood, cotton, and other plant-based materials. Natural cellulose synthesized by bacteria is referred to as bacterial cellulose (BC). BC has the chemical structure similar to that of plant-derived cellulose. In addition to microbial cells, BC is also produced by cell-free system [8], that also provide an advanced approach for in situ impregnation of nanoparticles into BC matrix [9]. However, it has the advantage of being naturally pure with no associated lignin and hemicellulose [10]. Moreover, it shows unique physical, chemical and mechanical properties, e.g., high porosity, high mechanical strength in the wet state, a high degree of crystallinity, high water holding capacity, and is non-toxic [10]. With such features, the use of BC is highly appealing in a wide range of applications. The synthesis of cellulose-based composites has been extensively studied, and many efforts have been focused on it. BC combined with gold nanoparticles (Au NPs) shows the excellent catalytic activity and stability [2]. By compositing BC with sodium alginate and silver sulfadiazine exhibited excellent antibacterial activities and good biocompatibility [3]. Nanocomposite of BC-titanium oxide (TiO2) was found efficient, stable and reusable for the removal of lead in environmental water [4] and constructed as composite membranes for combining photocatalytic and bioatalytic degradation of textile dye [5, 6]. Compositing BC with zinc-oxide nanoparticles were used as a new strategy for enhanced biomedical applications [7]. Silica (SiO2) particles, which are one as dispersed particles of the inorganic phase, are gaining considerable interest as incorporated components for improving the optical, mechanical and thermal properties of polymeric matrices [11, 12]. Recently, synthesized cellulose fiber-SiO2 hybrids with different techniques from different kinds of fiber and SiO2 sources have been reported [13–18]. For examples, BC-SiO2 nanocomposites were prepared by using the solution impregnation method [13], Stöber reaction [14], synthesized through a sol–gel process followed by freeze drying [15, 16], by ultrafast evaporative drying [17], and one-pot synthesis of cellulose surface modified by solvent exchange [18]. The aim of this work is to prepare novel BC–SiO2 composites. SiO2 NPs were extracted from rice husks, which is an agricultural by-product. BC–SiO2 composites aerogel was prepared by sol-gel method with some modification from the [16]. The obtained SiO2 NP with powder form was dissolved in NaOH to form (SiO3) prior to penetrate into BC matrix before forming SiO2. After preparation, the samples were characterized by means of x-ray diffraction (XRD), field emission scanning electron microscopy (FE-SEM), Fourier transform infrared (FTIR) spectroscopy and thermogravimetric analysis (TGA). These findings can be utilized as basic knowledge for the design and synthesis of BC and SiO2 composites for future environmental and biomedicine applications.
2. Materials and methods
2.1. Bacterial strains and growth conditions
A BC producing AAB strain, Gluconacetobacter xylinum BNKC 19, was used in this study. It was previously isolated from fermented plant juice [19]. A stock culture was activated by streaking it on Hestrin–Schramm (HS) agar medium and incubating at 30 °C for 3 days [20]. A pre-culture was prepared by transferring cells from a single colony into 3 ml of HS medium followed by incubation at 30 °C for 2–3 days until a thick pellicle was obtained.
2.2. BC production from the standard HS medium by Ga. xylinum BNKC 19
The pre-culture forming pellicles were transferred using an inoculating needle into a glass bottle containing 30 ml of sterile HS medium, and incubated at 30 °C for 7 days under a static condition. The thick pellicle BC was then collected and purified in a hot NaOH solution as previously reported [21].
2.3. Extraction of silica nanoparticles
In this work, rice husk ash (RHA) obtained from a brick factory was first rinsed with water several times to remove impurities. It was then burned at 700 °C for 3 h to extract silica. After heating to this temperature, its color was light gray. A sample of 15.0 g RHA was stirred into 200 ml of a 2.5 N sodium hydroxide (NaOH) solution. The resultant mixture was then heated in a covered beaker for 3 h with continuous stirring, after which it was filtered. The residue was then washed with 40 ml of 90 °C hot water. Next, the solution was cooled to room temperature. After that, a 10 M sulphuric acid (H2SO4) solution was added under constant stirring under controlled conditions until the solution pH reached 2. NH4OH was then added to raise the pH to 8.5 and the mixture was allowed to stand at room temperature for 3.30 h. The final product, a fine white powder, was stored in an air-tight container. To obtain nanostructured silica, 10.00 g of the resulting powder was refluxed in 6.0 M HCl for 4 h at 90 °C. To make it acid free, deionized water was used to repeatedly wash it. It was then dissolved in 2.5 M sodium hydroxide by stirring. H2SO4 was added at the rate of 2 ml min−1 until the solution reached pH 7.5–8.5. The precipitated silica was washed repeatedly with deionized water and then dried at 50 °C for 12 h in an oven.
2.4. BC–SiO2 nanocomposite preparation
BC pellicles with dimensions of 3 × 12 cm were soaked in the silica solution described in section 2.3 by dissolving them in 2.5 M NaOH at silica concentrations of 0, 3, 6, 9 and 12% (wt), covered by a plastic sheet. Then, it was mixed with a magnetic stirrer at 80 °C for 8 h, followed by immersion in 2 M sulfuric acid (H2SO4) for 8 h at ambient temperature. Finally, it was washed with distilled water to remove residual sulfuric acid.
2.5. Field emission scanning electron microscopy (FE-SEM)
The freeze-dried BC silica nanocomposites were subjected to surface analysis using field emission scanning electron microscopic (FE-SEM) imaging. FE-SEM was performed using a Helios NanoLab G3 CX unit (FEITM, Oregon USA) operating at 10 kV.
2.6. X-ray diffraction (XRD)
An x-ray diffractometer (Model X'Pert, Philips PANalytical, Netherlands) was utilized to analyze the crystallinity of the freeze-dried BC-silica CAs. The samples were scanned over a 2θ range of 5° to 40° with a step of 0.02θ.
2.7. Attenuated total reflection Fourier-transform infrared spectroscopy (ATR-FTIR)
Freeze-dried BC was crushed into powdered form before analysis. The FT-IR spectrum of the BC-silica CAs was characterized at wave numbers ranging from 4000 to 400 cm−1 using an FTIR spectrometer, a Bruker TENSOR27 (Bruker Optics, Germany), equipped with an ATR unit that was used for IR measurements.
2.8. Thermogravimetric analysis
Thermogravimetric analysis (TGA) by thermogravimetry/differential scanning calorimetry (TGA/DSC1 Analyzer, Mettler Toledo, Greifensee, Switzerland), was used to investigate the thermal stability of BC-silica CAs over the temperature range of 25 °C–550 °C, at a heating rate of 10 °C min−1 under a nitrogen atmosphere. The % mass loss, or mass loss fraction, was used as the degree of conversion in our calculations.
3. Results and discussion
Silica nanoparticle powders and BC were successfully prepared from rice husk ash and HS medium, respectively. Photographic images and selected SEM micrographs of silica nanoparticles and BC are shown in figures 1(a) and (b), respectively. A photographic image of the BC sample is shown in figure 1(b). Based on the SEM image in the inset of figure 1(b), it can be observed that prepared BC has a smooth and finer network of cellulose fibrils. SEM revealed a surface morphology of spheroid nanoscale silica particles with little agglomeration, in the inset of figure 1(a). The silica particles are porous with an average diameter ranging from 10–15 nm determined by SEM ( ) [22]. Preparation of BC-silica composites was carried using a permeation sol-gel method [16] with some modifications. The silica purified from rice husks was dissolved in 2.5 M NaOH to generate a precursor. The resulting cloudy solution had a turbidity that became more intense with higher silica contents.
) [22]. Preparation of BC-silica composites was carried using a permeation sol-gel method [16] with some modifications. The silica purified from rice husks was dissolved in 2.5 M NaOH to generate a precursor. The resulting cloudy solution had a turbidity that became more intense with higher silica contents.
Figure 1. Images of (a) silica nanoparticles from rice husk ash and (b) BC. Insets present their SEM images.
Download figure:
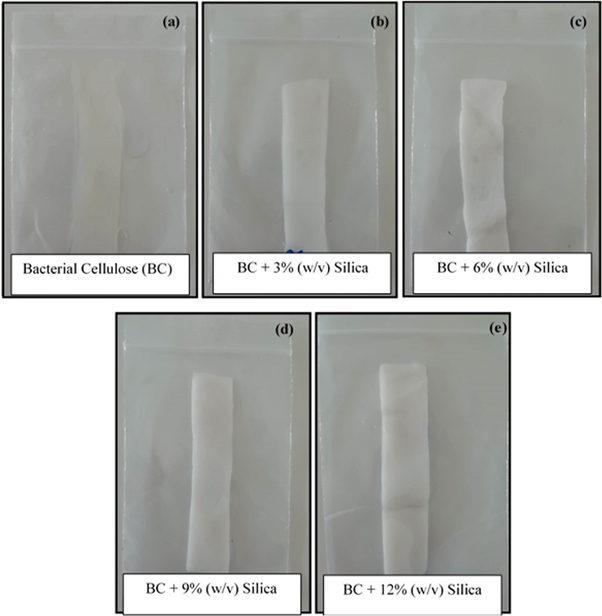
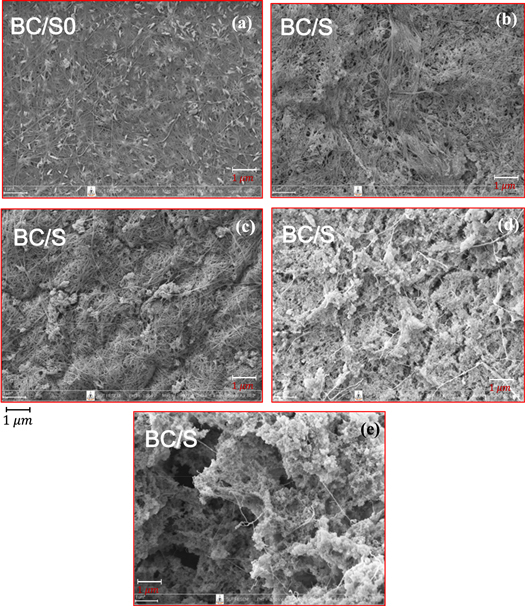
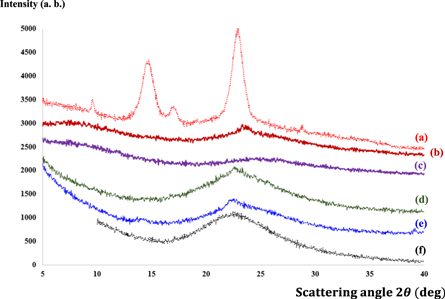
Standard image High-resolution imageThen, the silica precursor diffused into a three-dimensional (3D) BC matrix followed by gradual permeation of H2SO4 into the BC network to promote in situ condensation of the precursor to form a silica gel skeleton from the outside to the inside [16]. When it was immersed in 2 M H2SO4 for 8 h, the entire BC matrix became milky in color and lost transmittance at higher silica contents in an alkaline solution. The sample became harder as the silica in the BC matrix was converted into a rigid silica gel skeleton. This process deviated from those previously reported. As can be seen in figure 2, BC-silica composite shows progressively increasing contents of 0, 3, 6, 9 and 12% (w/v) of silica, represented by BC/S0, BC/S3, BC/S6, BC/S9 and BC/S12, respectively. A Na2SiO3 solution with various sizes of precipitated nanoparticulate silica was used to develop distinctive structures. Increased amounts of Na2SiO3 in the solution resulted in high levels of silica in the aerogel, concomitant with higher values of bulk density and surface area, but not porosity [16]. Figure 3 shows representative SEM micrographs of pure BC and BC-silica nanocomposites containing 3, 6, 9 and 12% (w/v) of silica. The SEM image of pure BC shows that, in the absence of silica nanoparticles, highly interconnected nanofibers formed a reasonably dense film with pores of irregular shape (figure 3(a)). As the distance between BC nanofibers was in micron scale, this matrix is allowed the formation of silica gel skeleton network properly. The micron-scale silica aerogel agglomerates are interconnected and constrained securely within the BC gel skeleton network, which prevents the CAs from being fragmented during freeze drying process. XRD analysis shows a broad peak of silica particles with a diffraction angle of 22–23o (figure 4). This confirms its amorphous and non-crystalline nature. XRD analysis shows a diffracted wave for the main peaks at around  and
and  that is associated with the BC structure. This is in good agreement with previously reported data [23]. In addition, diffraction peaks of BC were observed at around
that is associated with the BC structure. This is in good agreement with previously reported data [23]. In addition, diffraction peaks of BC were observed at around  (relate to the
(relate to the 
 and
and  planes, where 1α and 1β are cellulose phases [24] and at
planes, where 1α and 1β are cellulose phases [24] and at  (due to the plane of
(due to the plane of 
 ), shown in figure 4(a). However, with progressively increasing silica nanoparticle contents, the diffraction peaks of BC were gradually covered by a broad silica peak (at around
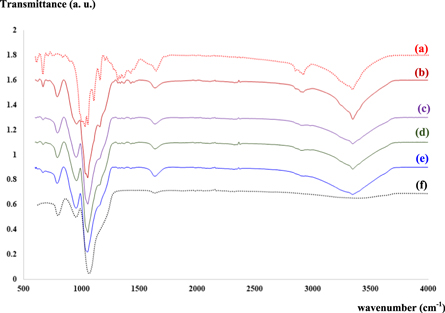
), shown in figure 4(a). However, with progressively increasing silica nanoparticle contents, the diffraction peaks of BC were gradually covered by a broad silica peak (at around  ). Additionally, no other diffraction peak was found other than those corresponding to peaks of BC and silica. Therefore, a combination and heat treatment of BC and silica, no new phases or new covalent bonds were produced as previously reported [25]. FTIR spectrum of silica nanoparticles at wavenumbers between 3000–4000 and at 1631 cm−1 (figure 5) implying to the H–O–H stretching and bending modes of adsorbed water were observed respectively [26].
). Additionally, no other diffraction peak was found other than those corresponding to peaks of BC and silica. Therefore, a combination and heat treatment of BC and silica, no new phases or new covalent bonds were produced as previously reported [25]. FTIR spectrum of silica nanoparticles at wavenumbers between 3000–4000 and at 1631 cm−1 (figure 5) implying to the H–O–H stretching and bending modes of adsorbed water were observed respectively [26].
Figure 2. Images of (a) BC/S0; (b) BC/S3; (c) BC/S6; (d) BC/S12 and (e) BC/S12.
Download figure:
Standard image High-resolution imageFigure 3. SEM micrographs of the surface morphology of (a) BC/S0, (b) BC/S3, (c) BC/S6, (d) BC/S9 and (e) BC/S12.
Download figure:
Standard image High-resolution imageFigure 4. XRD patterns of samples prepared with various silica concentrations in BC: (a) BC/S0, (b) BC/S3, (c) BC/S6, (d) BC/S9, (e) BC/S12 and (e) pure silica.
Download figure:
Standard image High-resolution imageFigure 5. FTIR patterns of BC-silica composite samples prepared with various non-solvent compositions of (a) BC/S0; (b) BC/S3; (c) BC/S6; (d) BC/S12 and (e) BC/S12.
Download figure:
Standard image High-resolution imageIn addition, the result of FTIR patterns for four representative BC-silica nanocomposites including BC/S3 (figure 5(b)), BC/S6 (figure 5(c)), BC/S9 (figure 5(d)) and BC/S12 (figure 5(e)) show some differences between the BC and BC-silica composites. As can be seen that the spectrum at wavenumbers of 1110–830 cm−1 (related to Si–O–Si vibrations) in figures 5(b), (c) were enhanced, while the peaks related to BC bands at 1400–1300 cm−1 and 2900–2700 cm−1 were clearly decreased which is in good agreement with previously reported work [13]. Addition a broadening of the 3500 cm−1 (O-H) band is observed. These may be due to hydrogen bonds formation between hydroxyls groups from bacterial cellulose and the silica nanoparticles [24], and may be related to strong chemical interactions between the BC and silica phases.
TGA observations of the thermal properties of BC-silica nanocomposites clearly show distinct decomposition temperatures of cellulose in each sample. Usually, thermal degradation of BC takes place in three phases: dehydration, depolymerization, and decomposition of glycosyl units, followed by the formation of a charred residue (Structural and physico-mechanical characterization of bio-cellulose produced by a cell-free system [27]. The weight loss at temperatures less than 100 °C can be ascribed to the volatilization of H2O, with subsequent removal of molecular fragments such as −OH and −CH2–OH. Two stages associated to thermal degradation of the BC membrane are observed at 285 °C and 380 °C (figure 6(a)) with almost complete weight loss can be seen at higher temperatures due to decomposition of cellulose chains. At this temperature range, processes such as depolymerization, dehydration, and breakdown of glycosidic bonds and the subsequent formation of CO2 and water are observed [24]. Similarly, other curves for the BC-silica nanocomposites show a first stage of superficial water evaporation from the membrane at temperature less than 150 °C followed by two stages of BC decomposition. It also can be observed that the thermal stability of the BC membranes around 300 °C is increased with addition of the silica. In later experiments, BC in nanocomposite aerogels did not decompose at temperatures of up to 550 °C which may indicate the effective interpenetrative of silica (SiO3) through BC matrix. This unique characteristic implies that silica had a stabilizing effect on polymeric cellulose [16, 25].
Figure 6. TGA curves of (a) BC/S0; (b) BC/S3; (c) BC/S6; (d) BC/S12 and (e) BC/S12.
Download figure:
Standard image High-resolution image4. Conclusions
The results of this study demonstrate that silica nanoparticles prepared from rice husks can be used as a precursor for sol-gel permeation after being dissolved in NaOH and subsequent a chemical crosslinking in the presence of H2SO4. SEM micrographs reveal that BC-silica retains the three-dimensional (3D) porous network structure of BC with nanometer sized silica particles well dispersed into the BC nano-fibril matrix. Increased silica loading into the precursor solution promotes formation of a silica skeleton that subsequently results in weight loss. This decomposition shifted to higher temperatures and the composite samples exhibited enhanced thermal stability. It is notable that the sample filled with 9 wt % of silica (BC/S9) exhibited the most improved thermal stability. This modified method to prepare CAs produced materials with some excellent properties that are beneficial for promoting utilization of materials derived from silica and agricultural by-products.
Acknowledgments
This work was financially supported by the Integrated Research Group for Energy and Environment (IRGEE), Khon Kaen University, Nong Khai Campus, Nong Khai. The authors acknowledge the facilities and support of the Faculty of Applied Science and Engineering, Nong Khai Campus, Khon Kaen University.