Abstract
The main challenge of the application of nanofluids for enhanced oil recovery (EOR) in oil-wet carbonate reservoirs is maintaining their stability under high-temperature and high-salinity which is normally seen in reservoir conditions. In this work, surface-modified TiO2 nanoparticles were synthesized by N-(2-Aminoethyl)−3-Aminopropyltrimethoxysilane (AEAPTMS) as a coupling agent for surface modification of nanoparticles. Zeta potential and dynamic light scattering analyses were used to evaluate the stability of the prepared nanofluids. Results indicated that nanofluids had high stability in 90 °C and 20 wt% salinity. The influence of nanofluids on wettability alteration of carbonate rocks was studied by measuring the contact angle value. Untreated rock samples were strongly oil-wet, with water advancing and receding contact angles of 165.1° and 166.2°, respectively. After treatment with 3wt% concentration of surface-modified TiO2 nanoparticles, the rock plate wettability changed to a water-wet state, with water advancing and receding contact angles of 37.6° and 48.2°, respectively. Suggested surface-modified TiO2 nanoparticles were more effective in wettability alteration of the rock surfaces compared to the un-modified TiO2 nanoparticles. Furthermore, higher concentration of nanoparticles, higher the ability of the nanoparticles on changing the rock wettability.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Nanotechnology is a new technology that makes applicable matters, devices and systems with nanosized materials (1–100 nm) [1]. By adding nanoparticles to injection water the oil production notably increases[2]. Nanotechnology can be applied for EOR in three major approaches: nanoemulsions, nanocatalysts, and nanofluids [3]. A nanofluid is a fluid in which nanoparticles are suspended in a base fluid such as brine or deionized water [4]. The injected nanoparticles into the reservoirs can improve oil recovery by wettability alteration, interfacial tension reduction, and disjoining pressure [5]. The main challenge in oil production is EOR from oil-wet carbonate reservoirs. About 33% of the discovered oil is trapped and cannot be produced from oil-wet carbonate reservoirs; wettability alteration from oil-wet to water-wet can be a great solution to enhance oil production in these reservoirs [6]. But the main challenge of the application of nanofluids for enhanced oil recovery in carbonate reservoirs is maintaining their stability under extreme conditions of high temperature and salinity [7]. In these conditions, nanoparticles may aggregate and plug pores that will result in permeability reduction and poor productivity [8]. These challenges are even more important in carbonate reservoirs because of their high salinity and multiplex porous system [6]. The usual salinity in carbonate reservoirs is about 90,000 ppm. Consequently, enhancement of the stability of the nanofluids is vital to prevent aggregation of particles [9]. There are some methods that can be used for this purpose, such as using surfactants in preparation of nanofluids and surface modification of the nanoparticles [10].
Ranka et al [11] reported an approach to graft zwitterionic polymers on polystyrene and silica nanoparticles. These nanoparticles showed great stability in a high-salinity and high-temperature conditions. But they didn't investigate the influence of these nanoparticles on the wettability of reservoirs. Bagaria et al [12] modified the surface of silica nanoparticles with polyacrylic acid (PAA) or polystyrene sulfonate (PSS) and the modified nanoparticles showed great stability in highly concentrated brine, but they didn't study the influence of temperature on the stability of the nanofluids and their application in wettability alteration. Yoon et al [13] fabricated oil-in-water Pickering emulsions that were stabilized by silica nanoparticles, poly (4-styrenesulfonic acid-co-maleic acid) sodium salt (PSS-co-MA), and dodecyl trimethylammonium bromide (DTAB). The prepared emulsions were injected into the Berea sandstone core samples and oil recovery was increased by 4% compared with water injection. Qiao et al [14] presented a hydrophobic SiO2 nanoparticles and used sodium dodecyl sulfate (SDS) for modification of the nanoparticles. Since SDS is water-soluble, it was mixed with silica nanoparticles very well, which was stable in an electrolyte solution. Zhu et al [15] examined the influence of SiO2 nanoparticles on the ability of HAHPAM solutions to enhance oil recovery under high-salinity and high-temperature conditions . The HAHPAM solution modified with SiO2 showed a better thermal stability than HAHPAM in brine without considering the effect of salinity in the environment. Zha et al [16] applied 3-aminopropyltrimethoxysilane (APTMS) and 3-Isocyanatopropyltrimethoxysilane (IPTMS) to enhance the durability of TiO2 nanoparticles at a fabric surface. Jang et al [4] used GPTMS, (3-Glycidoxypropyl) trimethoxysilane to modify the stability of silica nanoparticles in high-salinity and high-temperature conditions. They used these nanofluids to alter the wettability of carbonate rocks from oil-wet to water-wet and neutral-wet conditions. However, they didn't get a strong water-wet state that is more applicable for EOR.
This work aims to present an applicable nanofluid for wettability alteration of carbonate reservoirs from strongly oil-wet to water-wet condition at high- temperature and high-salinity conditions. The nanofluid is composed of titanium dioxide nanoparticles modified by N-(2-Aminoethyl)−3-Aminopropyltrimethoxysilane (AEAPTMS). The AEAPTMS is grafted to titanium dioxide to improve their hydrophilicity and increase stability of nanofluids. Contact angle measurement was used to investigate the application of the prepared nanofluids as an injection fluid for enhanced oil recovery to change the wettability of carbonate rocks. The stability of surface-modified TiO2 nanoparticles in nanofluids in high-salinity and high-temperature, similar to the temperature of an oil reservoir, is also examined. Morphology and particle size distribution and of nanoparticles were studied by scanning electron microscopy (SEM) and dynamic light scattering (DLS) methods.
2. Materials and methods
2.1. Materials and reagents
Titanium isopropoxide (TTIP, Ti (OCH(CH3)2)4, >98%), was purchased from Merck Company as the precursor for synthesizing TiO2 nanoparticles. AEAPTMS from Sigma-Aldrich was used for surface modification of nanoparticles. Absolute ethanol (EtOH, 99.5%, Merck) and nitric acid (HNO3, 65% in water, Merck) were employed as other components during the synthesis of nanoparticles. CaCl2 (93%) and NaCl (99%) were purchased from Sigma-Aldrich to obtain brine. NaCl to CaCl2 ratio was considered 80%: 20% in the brine formulation according to the ratio of API brine. Outcrop carbonate rock was used as the solid substrate in wettability alteration tests, and crude oil was used to create an oil-wet condition on the outcrop carbonate rock.
2.2. Characterization
Dynamic light scattering (DLS) was used to measure the size distribution of nanoparticles using the Nano ZS90 model from Malvern Instruments. SEM analysis was used to study the morphology of nanoparticles using a digital scanning electron microscope VEGA\\TESCAN model. XRD analysis was used to study the mineralogy of the synthesized nanoparticles using JOEL JDX-8030 x-ray diffrectometer with Cu Kα radiation. The XRD diffractograms were collected in the range of 2°–80° of 2θ. A Marvern Panalytical Zetasizer was used to measure zeta potential. The synthesized nanoparticles will also be characterized by Fourier-transform infrared spectroscopy (FTIR) using Perkin-Elmer spectrum RX1.
2.3. Preparation of un-modified and surface-modified titanium dioxide
When attractive forces between nanoparticles dominate repulsive forces, nanoparticles tend to aggregate. When the forces that repel particles are improved over the forces that attract particles, the particle aggregation is diminished and this will result in enhancing the stability of nanofluids [7]. In this study different amount of AEAPTMS, as a silane coupling agent, was used for surface modification of titanium dioxide nanoparticles to improve the stability of nanoparticles in nanofluids. AEAPTMS is an aminosilane which consists of three ethoxy (hydrolysable group) and one aminoethyl (nonhydrolysable group) [17]. In the surface functionalization of titanium dioxide by AEAPTMS, at first, AEAPTMS is hydrolyzed and silanols are obtained, according to figure 1(a); Then there is a condensation reaction between the obtained silanols and the OH groups on the titanium dioxide surface, as shown in figure 1(b) [18].
Figure 1. (a) Hydrolysis of AEAPTMS and (b) attachment of hydrolyzed AEAPTMS to the surface of TiO2.
Download figure:
Standard image High-resolution imageIn this study, un-modified and surface-modified TiO2 nanoparticles were synthesized via sol-gel process. To synthesize un-modified TiO2 nanoparticles, two solutions were prepared. 10 ml TTIP was added to 30 ml ethanol and stirred for 30 min (solution I). Furthermore, 3 ml HNO3 was added to 150 ml deionized water (solution II). For the hydrolysis reaction, solution II was added dropwise into solution I in about 4 h. Stirring was continued for another 2 h until a viscous solution was obtained. To evaporate the solvents, the obtained solution was placed at 100 °C for 24 h and after annealing at 400 °C for 1 h, un-modified titanium dioxide nanoparticles were obtained.
Surface-modified TiO2 nanoparticles was synthesized by adding AEAPTMS to the hydrolyzed TTIP solution and after stirring for two hours it was heated at 100 °C until the solvents were evaporated. Then, the obtained gel was calcined at 400 °C for 1 h. During this process, AEAPTMS was hydrolyzed and grafted to the surface of the TiO2 nanoparticles and surface-modified titanium dioxide nanoparticles were obtained. This process may enhance the stability of nanoparticles in the nanofluids and make nanoparticles more hydrophilic and applicable to change the wettability of the rock surface from strongly oil-wet to water-wet. The obtained nanoparticles were dispersed in a base fluid with a 10 wt% salt concentration, which is the highest salinity of formation water in an oil reservoir, and were used for stability tests and wettability alteration experiments.
2.4. Study of influence of different parameters in colloidal stability
The main factors in the preparation of nanofluids are the amount of silane, the nanoparticle concentration, temperature, and salinity. Considering these parameters and to investigate the colloidal stability, nanofluids were prepared in a certain amount of AEAPTMS, and varying concentrations of nanoparticles, salinity, and temperature. To prepare the nanofluids, synthesized nanoparticles were dispersed in a base fluid using a magnetic stirrer and an ultrasonic bath. Five different concentrations of nanofluids, 0.1, 0.5, 1, 2, and 3 wt%, were prepared to evaluate the effect of nanoparticles concentration on the stability of nanofluids, as well as their ability to change the rock wettability. To analyze the effect of temperature and salinity on the stability of nanofluids, the nanoparticles were dispersed in a brine solution with salt concentrations of 5, 10, 15, 20, 25, and 30 wt% and the temperature was set to 25 °C and 90 °C for 7 d. To investigate the colloidal stability, the particle size distribution and zeta potential were measured by DLS and zeta potential analyses, respectively. DLS measures the average size and size distribution of nanoparticles. The zeta potential is a very important factor to indicate the colloidal stability. The magnitude of the zeta potential shows the electrostatic repulsion between nearby particles with similar charge in a suspension. For small particles, a high zeta potential will verify the colloidal stability [19]. According to table 1, colloids with zeta potential more positive than 30 mV or more negative than 30 mV are considered to be moderately stable.
Table 1. Colloid stability based on zeta potential. Reproduced with permission from [20] Copyright © 2022 Thermal Science.
| Zeta potential (mV) | Stability behavior |
|---|---|
0 to 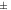 10 10 | Rapid coagulation or flocculation |
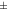 10 to 10 to 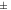 30 30 | Incipient instability |
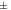 30 to 30 to 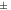 40 40 | Moderate stability |
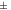 40 to 40 to 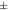 60 60 | Good stability |
| >61 | Excellent stability |
2.5. Experimental study of nanofluids
Wettability alteration from oil-wet to natural-wet or water-wet state appears to be a great approach to enhance oil production from oil-wet carbonate rocks [21]. Nanofluids with very low concentrations of nanoparticles can be applied for this purpose [21]. Here, the effect of the nanofluids, as an injection fluid, on the wettability alteration of carbonate rock are investigated. The outcrop carbonate rocks, which were not saturated with oil and initially had a water-wet state, were used in wettability alteration experiments. After cutting the rocks into small plates, 2 × 2 × 0.2 cm3, they were polished with an end face grinder to obtain smooth surfaces. Then, they were aged in crude oil at 90 °C for two weeks and deposition of polar compounds or asphaltenes from the crude oil onto rock surface created oil-wet conditions on the rock samples. The water droplet contact angle was measured to ensure the rock plates were oil-wet. Then, the oil-wet rock samples were aged in nanofluids with different nanoparticles concentrations for one day. To study the effect of nanofluids on wettability of carbonate rock, the water advancing and receding contact angles for each rock sample were measured. A 5–6 μl water droplet was injected into the rock samples using a micro-syringe. The rock samples were placed on a plate and it was inclined to the angle of 17°. The water droplet was injected onto the rock sample and when the water droplet started to move the contact angle was measured. An Attension Theta instrument was used for contact angle measurement.
3. Results and discussion
3.1. Characterization
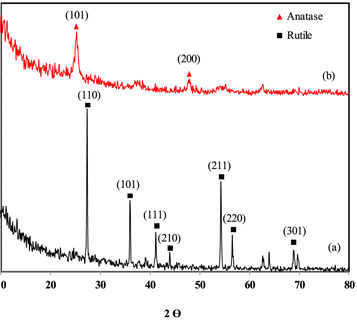
Figure 2 shows the XRD result of un-modified and surface-modified TiO2. As it can be observed, major crystalline phase of surface-modified and un-modified TiO2 nanoparticles were anatase and rutile, respectively. For un-modified TiO2 nanoparticles, figure 2(a), the diffractions were observed at 2θ angles around 27°, 36°, 41°, 44°, 54°, 56°, 62°, 64° and 69° corresponding to 110, 101, 111, 210, 211, 220, 002, 310 and 301 planes, respectively, which is related to rutile crystalline phase [22]. While, in XRD pattern of surface-modified TiO2, figure 2(b), diffractions were observed at 25° (101), 37° (004), 48° (200), 53° (105), 55° (211), 62° (204), 68° (116), 70° (220), 75° (215), indicate the anatase crystalline phase [23]. In surface-modified TiO2, phase transformation from anatase to rutile was suppressed, despite heat treatment at 600 °C, due to the formation of Ti-O-Si bond. This bond impedes titanium dioxide particles and prevents the nucleation of rutile phase [24].
Figure 2. XRD patterns of (a) un-modified and (b) surface-modified TiO2.
Download figure:
Standard image High-resolution imageThe SEM image of un-modified and surface-modified TiO2 nanoparticles were illustrated in figure 3. According to these images, these nanoparticles have spherical morphology. We used Image J software to evaluate the average size of nanoparticles. The results showed that the sizes of un-modified and surface-modified TiO2 nanoparticles are 42 ± 5 and 23 ± 2 nm, respectively.
Figure 3. SEM image of (a) un-modified and (b) surface-modified TiO2 nanoparticles.
Download figure:
Standard image High-resolution imageFTIR analysis, was used to identify the existent bonds in the synthesized nanoparticles. Figure 4(a) shows the FTIR spectrum of un-modified titanium dioxide nanoparticles, the strong peak in the region of 450 and 800 cm−1 is associated to the Ti-O bond and is the indication of TiO2 formation. In the FTIR spectrum of surface-modified titanium dioxide nanoparticles, as shown in the figure 4(b), the absorption peaks at1070, 770, 700 and 480 cm−1 are related to the Si–O, Si–C, Ti–O–Si and Ti–O bonds, respectively. In both FTIR spectrum, the in the range of 2840 and 3000 cm–1 is assigned to the C–H bonds and the peaks near 3400 and 1600 cm−1 are related to the hydroxyl bond (Ti–OH) and H–O–H bonds, respectively.
Figure 4. FTIR spectra of (a) un-modified TiO2 and (b) surface modified TiO2 over 450–4000 cm−1.
Download figure:
Standard image High-resolution image3.2. Stability study of the surface-modified TiO2 nanofluids
A stable nanofluid is the stable dispersion of nanoparticles with insignificant change in nanofluid such as changing the particle size with time. To evaluate the influence of AEAPTMS on the stability of nanofluids, surface-modified and un-modified TiO2 nanofluids were prepared according to table 2. Then DLS was used to determine the diameter of nanoparticles over time. To evaluate the influence of temperature on the stability of the prepared nanofluids, they were maintained at 25 °C and 90 °C. Equation (1) calculates the maximum theoretical number of moles of grafted silane per gram of TiO2 nanoparticle [4].
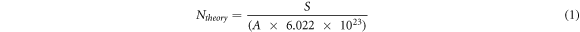
Where Ntheory is the maximum number of moles of grafted silane per gram of TiO2 nanoparticles, S is the specific surface area of TiO2 particles, and A is the surface area occupied by a silane molecule [4]. Here, S is 213 m2 g−1 and A is 32 Å2 and Ntheory is calculated as 1.105 mmol g−1.
Table 2. Preparation parameters of nanofluids.
| Un-modified TiO2 nanofluid | Surface-modifies TiO2 nanofluid | |
|---|---|---|
| Nanoparticles concentration | 0.5 wt% | 0.5 wt% |
| Base fluid | API brine (10 wt%) | API brine (10 wt%) |
| Surface modification with AEAPTMS | 0 | 5 ml |
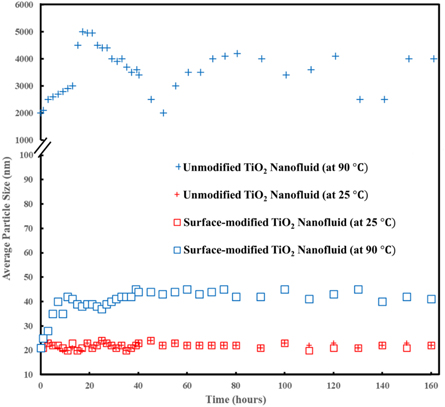
Figure 5 shows the average particle size of surface-modified and un-modified TiO2 nanoparticles versus time at 25 °C and 90 °C for 7 d. The results indicated that the average particle size of un-modified titanium dioxide nanoparticles remained nearly constant at 25 °C; however, it increased up to 5μm at 90 °C; this means that un-modified TiO2 nanoparticles aggregated and were unstable in these conditions. In the case of the surface-modified TiO2 nanofluids, the average particle size of nanoparticles remained relatively constant at 25 °C and nanofluids were stable; and at 90 °C nanoparticles grew up only to 43 nm at the first 10 h and after that, the diameter remained nearly constant and no more evolution of diameter was observed. The results showed that the surface-modified nanoparticles were stable in a nanofluid at high-temperature and high-salinity conditions of oil reservoirs; therefore, they can be a good option to be used as an injection fluid to increase the oil production.
Figure 5. Average particle size of surface-modified and un-modified titanium dioxide nanoparticles versus time.
Download figure:
Standard image High-resolution image3.3. Effect of the amount of AEAPTMS on the stability of nanofluid
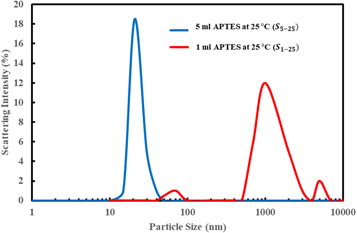
The amount of AEAPTMS as a silane coupling agent, in the surface modification of TiO2 nanoparticles, affects the stability of nanoparticles in nanofluids. For this purpose, different amounts of silane between 1 to 10 ml, were used in the synthesis of nanoparticles considering the calculated Ntheory. The concentration of nanoparticles was set to 0.5 wt%, which is mostly used in enhanced oil recovery experiments. The base fluids had 10 wt% and the temperature was set to 25 °C and 90 °C for 7 d. The prepared nanofluids were labeled as Sa−T and 'a' is the amount of AEAPTMS in the synthesis of nanoparticles and 'T' represents the temperature. Then, the particles size distribution and average particle size of nanoparticles were measured. All the nanofluids showed good stability after 7 at 25 °C. Figure 6 shows the particle size distribution of S1–25 and S5–25 samples. In the S1–25 sample, the sizes of particles are in the range of 40 nm to 7 μm and the average size of nanoparticles is 1 μm while in the S5–25 sample, the sizes of particles are in the range of 10 nm to 45 nm and the average size of nanoparticles is 20 nm. The average particle size of the S1–25 sample is about 50 times the average particle size of the S5–25 sample and this is an indication of aggregation between particles. Figure 7 shows the average particle size of the surface-modified TiO2 nanoparticles with different amounts of AEAPTMS at 25 and 90 °C. At 25 °C, the particle size does not change significantly with the amount of AEAPTMS at levels above 1 ml.
Figure 6. Particle size distribution of S1–25 and S5–25 samples.
Download figure:
Standard image High-resolution imageFigure 7. Average particle size of the surface-modified nanoparticles with various amounts of silane in the synthesis at 25 °C and 90 °C.
Download figure:
Standard image High-resolution imageIn the samples at 90 °C, the samples with less than 5 ml AEAPTMS were stable and for these samples, the average particle size was measured. The average particle size of S1–90, S2–90, S3–90, S4–90, and S5–90 samples are 23.2, 25.1, 24.7, 25.2, and 25 nm, respectively. According to this figure, at 90 °C the average particle size of the nanoparticles in the samples decreases with increasing the amount of AEAPTMS and the average particle size of the S1–90 sample is higher than the S5–90 sample; which indicates higher stability of S5–90 sample compared with the S1–90 sample. According to this results, the optimum amount of AEAPTMS to get the highest stability at 90 °C is 5 ml.
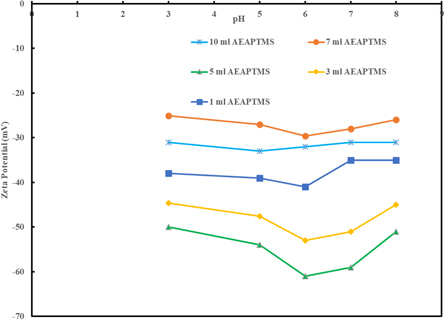
We also measured the zeta potential of nanofluids via a Malvern Panalytical Zetasizer, at 5 different pH values and after 7 d at 90 °C, to check the colloidal stability. Figure 8 shows the zeta potential of 0.5 wt% of surface-modified TiO2 nanofluids that were synthesized with different amounts of AEAPTMS in the feed. According to figure 8 and table 1, with increasing the amount of AEAPTMS up to 5 ml, the zeta potential became more negative and provided more stable nanofluids. Nanoparticles with 5 ml AEAPTMS are considered to have excellent stability. Besides, nanoparticles with more than 5 ml AEAPTMS were not stable at 90 °C and their zeta potential in different pHs verified that.
Figure 8. Zeta potential of 0.5 wt% of surface-modified TiO2 nanoparticles with the amount of AEAPTMS.
Download figure:
Standard image High-resolution imageHydrophilic surfaces are obtained by modifying the surface energy and surface roughness of the rock. Nanoparticles have high surface energy and by adsorption of nanoparticles on the rock surface, the wettability of the rock is changed.
According to table 1, particles with zeta potentials more negative than−30mV or more positive than +30 mV are considered to be stable. As you can observe in figure 8, the synthesized nanoparticles with 1, 3, 5, and 10 ml AEAPTMS have zeta potentials more negative than−30 mV. Therefore, they are considered to be stable with a negative surface charge.
Several studies have reported the zeta potential of calcite (CaCO3) surfaces [25, 26]. According to their results, in most ranges of pH, calcite is positively charged. Since nanoparticles have a negative surface charge and carbonate surface has a positive surface charge, nanoparticles are adsorbed on the rock surface. In presence of water, hydroxyl groups (Ti-OH), which are polar and hydrophilic, are formed. As a result, when TiO2 and surface-modified TiO2 nanoparticles are adsorbed on the carbonate rock surface, the wettability of rock is changed from oil-wet, with water contact angle value more than 90°, toward the water-wet state, with water contact angle value less than 90°.
3.4. Effect of nanoparticle concentration on the stability of nanofluids
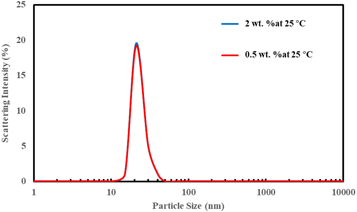
Concentration of nanoparticle is one of the main factors in the application of nanofluid as an injection fluid for EOR. In several reports, researchers have demonstrated that nanofluids with higher concentrations of nanoparticles are more efficient for changing the wettability of rock [10]. Here, nanofluids with five different concentrations of nanoparticles, 0.1, 0.5, 1, 2, and 3 wt%, were prepared; nanoparticles that were used in these experiments were obtained with 5 ml of AEAPTMS in the synthesis process, which is the optimum value according to the previous section. A brine solution with 10% salinity was used as the base fluid, and the temperature was set to 25 °C and 90 °C for 7 d. Figure 9 shows the particle size distribution of nanoparticles in nanofluids with 0.5 and 2 wt% particle concentrations at 25 °C. In both nanofluids, the same particle size distribution and average particle sizes were observed. Figure 10 shows the average particle size in different concentrations of nanoparticles at 25 °C and 90 °C. According to this figure, there is a small change in the average size of the nanoparticles at each temperature. This means the size of the nanoparticles in the nanofluids does not depend on their concentration.
Figure 9. Particle size distribution of samples with 0.5 and 2 wt% concentrations at 25 °C.
Download figure:
Standard image High-resolution imageFigure 10. Average particle size for different concentrations of nanoparticle at 25 °C and 90 °C.
Download figure:
Standard image High-resolution image3.5. Effect of concentration of salt on the stability of nanofluids
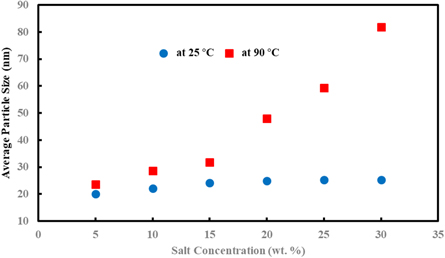
One of the changeless in applying nanofluids for EOR is high salinity of oil reservoirs, which may cause the nanofluids to be unstable. To analyze the effect of salinity on the stability of nanofluids, nanofluids with 0.5 wt% concentration of nanoparticles were prepared in a base fluid with salt concentration of 5, 10, 15, 20, 25, and 30 wt%, and the temperature was set to 25 °C and 90 °C for 7 d. Here, nanoparticles with 5 ml of AEAPTMS were used. Figure 11 shows the average size of nanoparticles in different concentrations of salt at 25 °C and 90 °C. At 25 °C the average particle size remains nearly 20 nm and it does not change significantly with increasing the salt concentration. However, at 90 °C the average particle size increases with increasing the salt concentration up to 80 nm; so, in higher temperature, the aggregation more often occurs between nanoparticles. For the purpose of EOR, nanoparticles with less than 100 nm are required and here, the particle size remained less than 100 nm in all nanofluid samples; so, the prepared nanofluids are great options for EOR.
Figure 11. Average size of nanoparticles for different concentrations of salt at 25 °C and 90 °C.
Download figure:
Standard image High-resolution image3.6. Surface wettability measurement
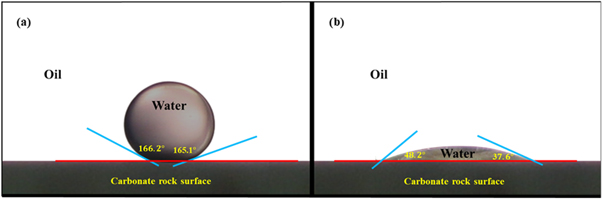
To study the influence of nanofluids on changing the wettability of carbonate rocks, water advancing and receding contact angles in the oil-water-solid three-phase system were measured. Three rock samples were aged in each nanofluid and contact angles were measured for each rock sample and the average value were reported as the contact angle. Nanofluids with five different concentrations of nanoparticles (0.1, 0.5, 1, 2, and 3 wt%.) were prepared to evaluate their effect on wettability of the rock samples. Figure 12 represents the water contact angles on the rock plates, before and after treatment with 3 wt% concentration of surface-modified TiO2 nanofluid. Water advancing and receding contact angles with the un-treated rock plate were 165.1° and 166.2°, respectively. This demonstrates that the carbonate rock before treatment with nanofluid was strongly oil-wet. Nanofluid with 3 wt% of particle concentration could change the wettability of rock to water-wet state with 37.6° and 48.2° advancing and receding contact angles, respectively.
Figure 12. water droplet contact angle on the rock surface (a) before and (b) after treatment with nanofluid with 3 wt% concentration of surface-modified TiO2 nanoparticles.
Download figure:
Standard image High-resolution imageFigures 13 and 14 show advancing and receding water droplet contact angles of the rock plates before and after treatment with nanofluids at different concentrations of nanoparticles; according to the results, water droplet contact angle decreases with an increase in nanoparticle concentration and nanofluids with higher concentrations are more effective in changing the wettability. Besides, in concentrations above 2 wt%, the wettability of the rock changes dramatically toward a more water-wet state. Also, the surface-modified titanium dioxide is more effective than un-modified titanium dioxide in altering the wettability of oil-wet carbonate rocks. Tables 3 and 4 present the results.
Figure 13. Water advancing contact angles of rock samples after treatment with different concentrations of nanofluids.
Download figure:
Standard image High-resolution imageFigure 14. Water receding contact angles of rock after treatment with different concentrations of nanofluids.
Download figure:
Standard image High-resolution imageTable 3. Water advancing contact angle of rock samples before and after treatment with different concentrations of un-modified and surface-modified TiO2 nanofluids.
| Nanofluid concentration (wt%) | Water advancing contact angle before and after treatment with surface-modified TiO2 nanoparticles (Degree) | Water advancing contact angle before and after treatment with un-modified TiO2 nanoparticles (Degree) | ||||
|---|---|---|---|---|---|---|
| Before treatment | After treatment | Difference | Before treatment | After treatment | Difference | |
| 0.1 | 165.1 | 131.2 | 33.9 | 165.1 | 145.1 | 20 |
| 0.5 | 110.6 | 54.5 | 131.2 | 33.9 | ||
| 1 | 89.1 | 76 | 115.2 | 49.9 | ||
| 2 | 41 | 124.1 | 87.2 | 77.9 | ||
| 3 | 37.1 | 128 | 80.4 | 84.7 | ||
Table 4. Water receding contact angle of rock samples before and after treatment with unmodified and surface modified TiO2 nanofluids with different particle concentrations.
| Nanofluid concentration (wt%) | Water receding contact angle before and after treatment with surface-modified TiO2 nanoparticles (Degree) | Water receding contact angle before and after treatment with unmodified TiO2 nanoparticles(Degree) | ||||
|---|---|---|---|---|---|---|
| Before treatment | After treatment | Difference | Before treatment | After treatment | Difference | |
| 0.1 | 166.2 | 133.1 | 33.1 | 166.2 | 145.1 | 21.1 |
| 0.5 | 115.4 | 50.8 | 131.2 | 35 | ||
| 1 | 100 | 66.2 | 115.2 | 51 | ||
| 2 | 64.1 | 102.1 | 87.2 | 79 | ||
| 3 | 48.2 | 118 | 80.4 | 85.8 | ||
4. Conclusions
Here, we synthesized surface-modified TiO2 nanoparticles using N-(2-Aminoethyl)−3-Aminopropyltrimethoxysilane (AEAPTMS). Then, the obtained nanoparticles were used to prepare nanofluids. The performance and stability of the nanofluids were investigated to prove their applicability in high-temperature and high-salinity carbonate reservoirs. The results showed that the optimum amount of AEAPTMS is 5 ml for 10 ml of TTIP and the stability of nanofluids does not affect by the concentration of nanoparticles. At 90 °C and salinities above 30 wt%, when the amount of AEAPTMS was 5 ml, nanofluids were stable. The nanofluids could alter the wettability of carbonate rock surfaces from strongly oil-wet to water-wet with a concentration of 0.5% wt.
In conclusion, the prepared nanofluids showed a great stability in high-salinity and high-temperature conditions and they were effective in wettability alteration of carbonate rocks. Consequently, this nanofluid is a great option to be injected in carbonate reservoirs for EOR.
Data availability statement
All data that support the findings of this study are included within the article (and any supplementary files).