-
PDF
- Split View
-
Views
-
Cite
Cite
Jean-François Le Galliard, G. Gundersen, H.P. Andreassen, N.C. Stenseth, Natal dispersal, interactions among siblings and intrasexual competition, Behavioral Ecology, Volume 17, Issue 5, September/October 2006, Pages 733–740, https://doi.org/10.1093/beheco/arl002
Close - Share Icon Share
Abstract
According to the classical model of a polygynous mating system, male-biased dispersal is a consequence of inbreeding avoidance and sexual asymmetries in competition. However, kin cooperation can change the costs and benefits of dispersal to each sex and may also select for philopatry in females. Here, we report from an experimental study designed to tease apart the effects of competition, cooperation, and inbreeding avoidance on natal dispersal in juvenile root voles (Microtus oeconomus). We manipulated the presence of opposite-sex littermates and tested how interactions among siblings influence dispersal and sexual maturation. We also manipulated the juvenile sex ratio to compare the strength of intrasexual versus intersexual competition. Natal dispersal was unrelated to the juvenile sex ratio, females aggregated in space, and there was a consistent spatial overlap among sisters. Males dispersed more in the absence of their sisters, resulting in stronger spatial segregation between sexes, than in the presence of their sisters. Thus, natal dispersal did not reduce the risks of interactions with siblings and intrasexual competition. We suggest that kin clusters in females function as a defense against aggressive or infanticidal behavior by unfamiliar males.
Natal dispersal is a fundamental demographic process influencing the dynamics, distribution, and genetics of natural populations (Stenseth and Lidicker 1992). Sex-biased natal dispersal is common in vertebrates, and it has been proposed that the evolution of sex-biased natal dispersal is molded by interactive effects of kin competition, mating system, and inbreeding avoidance (Greenwood 1980; Moore and Ali 1984; Pusey 1987; Perrin and Mazalov 2000). In polygynous species, such as many mammals with female-defense mating systems, female offspring usually disperse less or shorter distances than males (Dobson 1982). Greenwood (1980) hypothesized that sexual asymmetries in the degree of competition explain the male-biased natal dispersal in these species. Greenwood suggested that competition for resources in females induce benefits of philopatry through acquaintance with the natal territory and thus promotes territorial defense of resources from the natal area in this sex (Perrin and Mazalov 2000). Furthermore, in female-defense polygyny, males have a higher potential reproductive rate than females, and their fitness should be strongly limited by the availability of mates (Emlen and Oring 1977). Thus, young males should be forced to disperse from their natal area to find mates and therefore avoid inbreeding (Dobson 1982; Perrin and Mazalov 2000; Perrin and Goudet 2001).
Although Greenwood (1980) realized that intrasexual competition and inbreeding avoidance influence sex-biased dispersal, he also suggested that philopatry could interact with cooperative behaviors. When cooperation benefits one sex more than the other, kin cooperation selects for strong philopatry in the cooperative sex, whereas the other sex should disperse to avoid the costs of inbreeding (Perrin and Goudet 2001; Perrin and Lehmann 2001). In polygynous mammals, social structures are widespread, and cooperative behaviors often involve females. At one end of the range of social structures, females engage in altruistic interactions, such as vigilance behaviors, collective foraging, or cooperative breeding (Perrin and Goudet 2001). However, even in less social species, females may tolerate relatives to a large extent and collectively defend natal resources against strangers (e.g., Mappes et al. 1995). Thus, kin cooperation among females has the potential to induce female-biased philopatry in polygynous mating systems.
More recently, our understanding of natal dispersal as a behavioral process has changed (Stamps 2001). After the accumulation of empirical data, the idea of a rigid sex-biased dispersal has been challenged, and evidences that natal dispersal is a plastic behavior have grown (Clobert et al. 2004). Thus, the interest in natal dispersal has shifted to identifying the social and environmental cues that trigger movements, which might ultimately inform us on the evolutionary causes of dispersal (Stamps 2001; Clobert et al. 2004). Competition, cooperation, and inbreeding avoidance should be major social factors underlying plasticity in natal dispersal in polygynous mating systems. However, few studies have investigated the joint effects of these social factors on natal dispersal (reviewed by Lambin et al. 2001), especially as they pertain to interactions among siblings (Lambin 1994b). Ideally, such studies should rely on experimental manipulations that tease apart the various social causes of natal dispersal (Shields 1987).
Populations of microtine rodents (Arvicolidae) represent ideal study systems to understand the effects of competition, cooperation, and inbreeding avoidance on sex-biased natal dispersal (Stenseth and Lidicker 1992). The typical mating system of microtine rodents is polygyny, males disperse further and more rarely reach sexual maturity within their natal home range than females (Boonstra et al. 1987). In most species investigated so far, kin recognition depends on familiarity and preweaning association (Paz y Miño et al. 2002 and references therein). Furthermore, microtine rodents exhibit substantial plasticity in space use, territorial behavior, and natal dispersal (e.g., Lambin 1994a). This group is also characterized by extensive intraspecific variations in age at sexual maturity in response to social factors (Stenseth and Lidicker 1992; Gundersen and Andreassen 1998; Solomon 2003). In female-defense polygynous species, females compete for space and food resources, whereas males compete for mating partners (Ostfeld 1985; Ims 1987). Sociality within female kin groups has been described in several species (McGuire et al. 1993; Mappes et al. 1995; Lambin and Yoccoz 1998), and kin clusters in females seem to be often associated with defense against aggressive unfamiliar conspecifics, in particular against infanticidal males in Microtus species (Agrell et al. 1998; Ebensperger 1998).
Here, we investigate space use and sexual maturation in juvenile root voles (Microtus oeconomus), a polygynous microtine rodent species with male-biased natal dispersal (Lambin et al. 1992; Gundersen and Andreassen 1998). We evaluated the effects of competition, cooperation, and inbreeding avoidance on natal dispersal and sexual maturation. According to the classical female-defense polygyny model (Greenwood 1980), intrasexual competition is more important than intersexual competition, so that juvenile root voles should avoid competition with same-sex conspecifics. In particular, juveniles should avoid competition with same-sex relatives if kin competition is important. Furthermore, juveniles should avoid spatial overlap with opposite-sex relatives if inbreeding avoidance is involved. However, when female relatives benefit from space sharing against the threat of infanticidal males (Andreassen and Gundersen 2006), female relatives should cluster in space and avoid unrelated males. To tease apart these social factors, we first manipulated the presence of opposite-sex littermates at the natal site and tested how interactions among siblings influence dispersal and sexual maturation. We also manipulated the juvenile sex ratio at the natal site to compare the strength of intrasexual versus intersexual competition. We created male-biased and female-biased populations, setting the conditions for strong intrasexual competition in males and in females, respectively. We used fenced landscapes consisting of a release habitat patch, an immigration habitat patch, and a surrounding barren matrix (Gundersen and Andreassen 1998). Newly weaned voles were introduced in release habitat patches, and their survival, body growth, maturation, and space use during the critical life stage for territory acquisition were measured.
MATERIALS AND METHODS
Model species
The root vole (M. oeconomus) is a small and sexually dimorphic microtine rodent with adult males approximately 30% larger than females (Bondrup-Nielsen and Ims 1990). Root voles can be regularly found in patchy, agricultural landscapes where they require humid grasslands for cover and food (Tast 1966). In our field site located in Evenstad, Hedmark County, southeast Norway (250 m above sea level, 61°25′N, 11°04′E), the life cycle of the root vole is seasonal, and most reproduction occurs from spring to late autumn. The litter size ranges from 1 to 11 pups, and lactation lasts a minimum of 15 days. Females can conceive as early as 18 days of age in spring (G Gundersen, personal observation). During summer, year-born offspring typically reach sexual maturity before the age of 1 month, and natal dispersal occurs at the age of puberty (Andreassen and Ims 2001). Natal dispersal bears some costs, such as increased exposure to predation during transience (Andreassen and Ims 2001) and competition with residents during settlement (Gundersen et al. 2002).
Experimental system
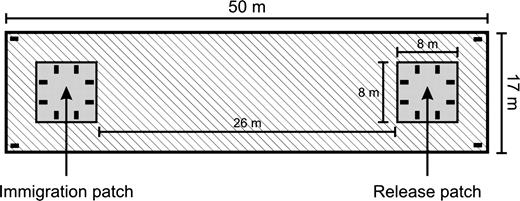
The experiment took place between 16 June and 7 October 2004 at Evenstad Research Station. The experimental area consisted of 6 plots fenced with vole-proof barriers and each measuring 50 × 17 m. In May 2004, we created 2 habitat patches of tall and dense meadow vegetation in each of the 6 plots by mowing and herbiciding the surroundings (see Figure 1). Herbiciding of the nonhabitat areas was done every other week to prevent settlement of voles in these areas. The distance between habitat patches was 26 m, and each habitat patch measured 8 × 8 m, which is approximately the same size as one home range core area of breeding females at Evenstad (Andreassen et al. 1998). To prevent predation, the entire study area was surrounded by a chicken wire fence 1.5 m high supplied with an electric wire and covered by a net extending approximately 2 m above ground.
Habitat configuration and trap locations (filled boxes) of 1 of the 6 study plots. Gray shaded areas are habitat patches, whereas the hatched areas are barren matrix habitats.
Experimental procedures
Root voles used in the present experiment were obtained by breeding one parent originating from the Valdres population and one parent from the Finse population, southern Norway. Breeding pairs were kept in a room at the Animal Division of the University of Oslo, with separate cages (60 × 30 × 30 cm) for each pair of root voles. Photoperiod (16:8 h light:dark), temperature (14 °C), food (laboratory pellets, oats, and hay), and daily care were standardized according to the procedures used by the Animal Division. To obtain animals for release at the right age throughout our study, one breeding group of 20 pairs was initiated 41 days before the start of the field experiment and a second breeding group of 20 pairs was initiated 11 days later. Individuals from pairs that failed to produce pups were swapped to produce new breeding pairs and synchronized with one of the breeding groups. The exact day of birth was determined by checking the cages several times per week, and the number of pups at the day of birth was counted. The day prior to release, the number of weaned offspring was counted, and we individually marked all animals by toe clipping. Offspring (n = 456) were sexed, weighed (to the nearest 0.1 g), and measured for head width (to the nearest 0.01 mm) by the same person.
Each experimental trial started with the release of 4 offspring within one habitat patch of each of the 6 study plots (see Figure 1). We released 4 animals to avoid variations due to effects of vole density on emigration and settlement and to simulate realistic levels of competition for space (Gundersen et al. 2002). Young voles were selected for release from litters with more than 4 offspring and assigned to 1 of the 4 treatments: “2-sex and all kin” (2 females and 2 males, all from the same litter), “2-sex and mixed kin” (2 females from the same litter and 2 males from another litter), and “1-sex and mixed kin” with males (2 pairs of male siblings) or females (2 pairs of female siblings). The 4 treatments are from now referred to as FFMM, FF‖MM, MM‖MM, and FF‖FF, respectively. Treatments were matched for offspring age, body mass, and head width, as well as for litter size and litter sex ratio (i.e., the sex ratio of the full litter from where experimental animals were selected). To ensure independence between treatments and plots, we interspersed the spatial location of treatments during each experimental trial and changed the location of treatments between trials. To avoid dependence between treatments and breeding pair identity, we distributed randomly the families among treatments throughout the study. We performed 10 trials, consisting of 120 young females, 120 young males, and 15 replicates per treatment group.
In the evening before the release day, we introduced siblings of the same release plot inside cages and placed the cages in the center of the release habitat patch (Figure 1). Cages allowed visual, auditory, and olfactory contacts between individuals in the 2 cages (for treatment FF‖MM, MM‖MM, and FF‖FF) and the outside. Cages were opened early in the morning and stayed at the release point during the whole trial. Andreassen and Ims (2001) found that juvenile root voles start to disperse between neighboring patches around the mean age of 25.3 days ([23.3, 27.3] 95% confidence interval [CI]). To conduct our experiment during the critical life stage for natal dispersal, animals were therefore left undisturbed during a settlement period of 10 days and recaptured around the mean age of 32 days. Thereafter, we removed all surviving voles with Ugglan live traps. Eight live traps were located on the edge of each patch and 2 by the fences (fence traps, see Figure 1). Traps, baited with carrots and wholegrain oats, were activated at midnight for 24 h with trap checks every sixth hour. Using this procedure, we removed 91%, 6%, and 3% of the surviving voles during the first, second, and third trap check, respectively. The same person recorded individual identity, trap station, body mass, and head width for each trap event.
Statistical analyses
We captured and removed all surviving individuals at the end of each trial (100% trappability) and hence could determine the individual's fate (alive vs. dead) without statistical uncertainty. Body growth was measured for body mass and head width as the increase in mass and head width from release to recapture, respectively. Voles were recorded as having reached sexual maturity if males had scrotal testes and if females had perforated vagina. “Natal dispersal status” was defined according to the location of individuals at removal (Gundersen and Andreassen 1998). Individuals removed from the release patch were defined as “residents,” whereas individuals removed from the immigration patches were called “immigrants.” Only 6 individuals in 224 captures were trapped along the fences and thus had an ambiguous trapping location. To assign these animals to one patch, we placed 4 dishes with dyed oat porridge in each patch 24 h before the start of each trapping and examined patch-specific dyes in the feces of trapped animals (Hovland et al. 1999). All animals captured in fence traps were assigned to the nearest habitat patch using this technique, which suggests that they were captured during occasional sallies out of their main foraging patch. In addition to dispersal status, we also defined 2 measures of spatial overlap among conspecifics. We defined “intrasexual littermate overlap” as the probability of having a same-sex littermate in the same patch. This was done by estimating for each individual whether it was trapped in the same patch as a littermate of the same sex or not. For FFMM and FF‖MM treatments, we further estimated “intersexual overlap” as the probability that the individual was trapped in the same patch as an opposite-sex individual.
We compared survival, growth, sexual maturation, and space use (dispersal and the 2 overlap frequencies) among 3 treatment levels: 1) 2-sex and all-kin group (FFMM), 2) 2-sex and mixed-kin group (FF‖MM), and 3) the 1-sex groups (FF‖FF and MM‖MM). The full models included the fixed effects of treatment groups (categorical factor, 3 levels), sex, season, and their 2-way interactions. We modeled seasonal changes with a linear effect of study trial after checking for nonlinearity. We used replicate within treatment as a nested random factor. In addition, we added the random effects of breeding pair and plot identity. Binary responses (survival, dispersal, and maturation) were modeled with the GLIMMIX macro of SAS version 8.2 (Littell et al. 1996) using a logit link and a binomial error term. The goodness of fit of the models was checked with Pearson chi-square tests and satisfied in all cases. Body growth (body mass and head width) was analyzed with maximum likelihood–based mixed models using the SAS MIXED procedure. Assessments of model assumptions (normality and homogeneity of residuals) were satisfactory in all cases. For each response, a final model was selected after backward elimination of nonsignificant terms (P > 0.05).
To further test if voles aggregated in space, we compared the number of animals trapped within each patch with a random distribution generated by Monte Carlo simulations (Gundersen and Andreassen 1998). The number of surviving animals was randomly allocated to the 2 patches for all replicates (null hypothesis), and the number of patches inhabited by 0, 1, 2, 3, or 4 voles was counted. A total of 5000 independent simulations was used to generate a frequency distribution of patch density under the null hypothesis of random settlement. We compared the observed values with the central 95% interval (i.e., the 2.5–97.5% quantiles) of the simulated random distribution.
RESULTS
Characteristics at release
Neither did body mass and head width differ between treatments at the start of the study nor did litter size, litter sex ratio, or age of individuals (all P > 0.67, except for litter sex ratio where treatment effect had P = 0.12). On average, voles were aged 22.2 days ± 3.02 standard deviation (SD) when released, weighed 16.09 g ± 3.65 SD, and had a head width of 12.37 mm ± 0.68 SD. The mean litter size was 5.97 pups ± 1.29 SD, and the average litter sex ratio was balanced (percent males = 48 ± 13 SD).
Survival and growth
Of the 240 released offspring, 224 individuals survived during the course of the field study. Survival probability was high (mean = 0.97 [0.95, 0.98] 95% CI) and did not vary with treatment group, sex, or season (all P > 0.72). The body growth of animals was not influenced by treatment group, neither for body mass (treatment: F2,56 = 0.06, P = 0.94; treatment × sex: F2,128 = 0.83, P = 0.44) nor for head width (treatment: F2,56 = 1.98, P = 0.15; treatment × sex: F2,128 = 0.41, P = 0.66). Body growth was higher in males than in females (body mass: F1,129 = 37.77, P < 0.0001, contrast = 5.87 g ± 0.95 standard error [SE]; head width: F1,130 = 18.20, P < 0.0001, contrast = 0.33 mm ± 0.08 SE) and decreased throughout the season (body mass: F1,129 = 57.67, P < 0.0001, slope = −1.13 g per trial ± 0.14 SE; head width: F1,130 = 19.72, P < 0.0001, slope = −0.19 mm per trial ± 0.02 SE). The seasonal decrease in body mass was more pronounced in males than in females (F1,129 = 37.77, P < 0.0001, contrast = −0.36 g per trial ± 0.15 SE).
Effects of treatments on spatial distribution and maturation
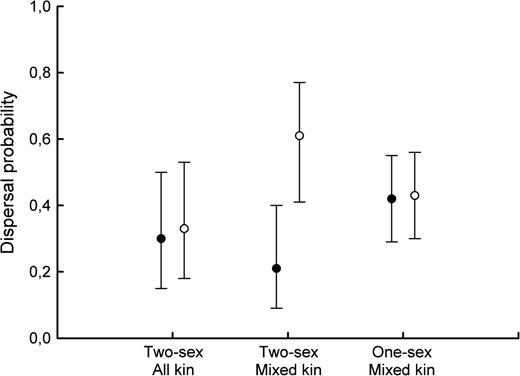
The dispersal probability was on average 0.42 (SE = 0.08). A significant interaction between treatment group and sex influenced dispersal probability (Figure 2, Table 1). There was no difference in dispersal probability between males and females from the FFMM and 1-sex groups (logit contrast = 0.17, SE = 0.74, P = 0.82). However, males dispersed more than females in FF‖MM (Figure 2, Table 1). This sex-specific response to the presence of opposite-sex siblings resulted in contrasted patterns of intersexual overlap between treatments, with intersexual overlap frequency being stronger in all-kin than in mixed-kin groups (Table 2). Intrasexual littermate overlap frequency did not differ among treatments, neither for males nor for females (Table 2). Independent of treatment, the overlap frequency between male siblings was 0.57 ([0.37, 0.75] 95% CI) as expected if brothers settled randomly (t = 0.75, degrees of freedom [df] = 38, P = 0.46). On the contrary, female siblings overlapped more often than expected at random (mean = 0.79 [0.49, 0.94] 95% CI; t = 2.00, df = 41, P = 0.05). When analyzing spatial overlap separately in the release and immigration patches, we found that female siblings overlapped significantly in the release patch (mean = 0.86 [0.61, 0.96] 95% CI, P = 0.01) but not in the immigration patch (P = 0.94).
Effects of the treatments on dispersal probability. Least square means (with 95% CI) of the dispersal probability of female (filled circles) and male (open circles) root voles per treatment are presented (model described in Table 1).
Selected model describing dispersal status (resident or immigrant) in root voles from the 3 treatment groups
| Factors . | Estimates ± SE . | t Values . | F statistics . |
|---|---|---|---|
| Fixed effects | |||
| Intercept | −0.30 ± 0.33 | −0.93, P = 0.36 | — |
| Treatment | |||
| FFMM | −0.46 ± 0.55 | −0.83, P = 0.41 | F2,56 = 0.74, P = 0.48 |
| FF‖MM | 0.76 ± 0.54 | 1.41, P = 0.16 | |
| FF‖FF and MM‖MM | 0 | — | |
| Sex | |||
| Females | −0.034 ± 0.47 | −0.07, P = 0.94 | F1,162 = 4.53, P = 0.03 |
| Males | 0 | — | |
| Treatment × sex | |||
| Females FFMM | −0.10 ± 0.74 | −0.14, P = 0.89 | F2,162 = 3.28, P = 0.04 |
| Females FF‖MM | −1.80 ± 0.75 | −2.41, P = 0.02 | |
| FF‖FF and MM‖MM | 0 | — | |
| Random effects | |||
| Replicate (treatment) | σ2 = 0.60 ± 0.36 | Z = 1.65; P = 0.05 |
| Factors . | Estimates ± SE . | t Values . | F statistics . |
|---|---|---|---|
| Fixed effects | |||
| Intercept | −0.30 ± 0.33 | −0.93, P = 0.36 | — |
| Treatment | |||
| FFMM | −0.46 ± 0.55 | −0.83, P = 0.41 | F2,56 = 0.74, P = 0.48 |
| FF‖MM | 0.76 ± 0.54 | 1.41, P = 0.16 | |
| FF‖FF and MM‖MM | 0 | — | |
| Sex | |||
| Females | −0.034 ± 0.47 | −0.07, P = 0.94 | F1,162 = 4.53, P = 0.03 |
| Males | 0 | — | |
| Treatment × sex | |||
| Females FFMM | −0.10 ± 0.74 | −0.14, P = 0.89 | F2,162 = 3.28, P = 0.04 |
| Females FF‖MM | −1.80 ± 0.75 | −2.41, P = 0.02 | |
| FF‖FF and MM‖MM | 0 | — | |
| Random effects | |||
| Replicate (treatment) | σ2 = 0.60 ± 0.36 | Z = 1.65; P = 0.05 |
Treatment groups are 2-sexes and all-kin group (FFMM), 2-sexes and mixed-kin group (FF‖MM), and the 1-sex groups (FF‖FF and MM‖MM). Results are from a mixed-effects logistic regression (see text for details).
Selected model describing dispersal status (resident or immigrant) in root voles from the 3 treatment groups
| Factors . | Estimates ± SE . | t Values . | F statistics . |
|---|---|---|---|
| Fixed effects | |||
| Intercept | −0.30 ± 0.33 | −0.93, P = 0.36 | — |
| Treatment | |||
| FFMM | −0.46 ± 0.55 | −0.83, P = 0.41 | F2,56 = 0.74, P = 0.48 |
| FF‖MM | 0.76 ± 0.54 | 1.41, P = 0.16 | |
| FF‖FF and MM‖MM | 0 | — | |
| Sex | |||
| Females | −0.034 ± 0.47 | −0.07, P = 0.94 | F1,162 = 4.53, P = 0.03 |
| Males | 0 | — | |
| Treatment × sex | |||
| Females FFMM | −0.10 ± 0.74 | −0.14, P = 0.89 | F2,162 = 3.28, P = 0.04 |
| Females FF‖MM | −1.80 ± 0.75 | −2.41, P = 0.02 | |
| FF‖FF and MM‖MM | 0 | — | |
| Random effects | |||
| Replicate (treatment) | σ2 = 0.60 ± 0.36 | Z = 1.65; P = 0.05 |
| Factors . | Estimates ± SE . | t Values . | F statistics . |
|---|---|---|---|
| Fixed effects | |||
| Intercept | −0.30 ± 0.33 | −0.93, P = 0.36 | — |
| Treatment | |||
| FFMM | −0.46 ± 0.55 | −0.83, P = 0.41 | F2,56 = 0.74, P = 0.48 |
| FF‖MM | 0.76 ± 0.54 | 1.41, P = 0.16 | |
| FF‖FF and MM‖MM | 0 | — | |
| Sex | |||
| Females | −0.034 ± 0.47 | −0.07, P = 0.94 | F1,162 = 4.53, P = 0.03 |
| Males | 0 | — | |
| Treatment × sex | |||
| Females FFMM | −0.10 ± 0.74 | −0.14, P = 0.89 | F2,162 = 3.28, P = 0.04 |
| Females FF‖MM | −1.80 ± 0.75 | −2.41, P = 0.02 | |
| FF‖FF and MM‖MM | 0 | — | |
| Random effects | |||
| Replicate (treatment) | σ2 = 0.60 ± 0.36 | Z = 1.65; P = 0.05 |
Treatment groups are 2-sexes and all-kin group (FFMM), 2-sexes and mixed-kin group (FF‖MM), and the 1-sex groups (FF‖FF and MM‖MM). Results are from a mixed-effects logistic regression (see text for details).
Estimates of intrasexual littermate overlap frequencies and intersexual overlap frequencies in each treatment group and the F statistics of the differences between treatment groups
| Category . | Estimates and 95% CI . | F statistics . |
|---|---|---|
| Intrasexual littermate overlap: males | ||
| FFMM | 0.63 (0.25, 0.90) | F2,39 = 0.08, P = 0.92 |
| FF‖MM | 0.56 (0.21, 0.86) | |
| MM‖MM | 0.55 (0.25, 0.83) | |
| Intrasexual littermate overlap: females | ||
| FFMM | 0.75 (0.18, 0.97) | F2,36 = 0.05, P = 0.95 |
| FF‖MM | 0.79 (0.24, 0.98) | |
| FF‖FF | 0.83 (0.33, 0.98) | |
| Intersexual overlap | ||
| FFMM | 0.85 (0.72, 0.94) | F1,108 = 3.23, P = 0.07 |
| FF‖MM | 0.70 (0.57, 0.81) |
| Category . | Estimates and 95% CI . | F statistics . |
|---|---|---|
| Intrasexual littermate overlap: males | ||
| FFMM | 0.63 (0.25, 0.90) | F2,39 = 0.08, P = 0.92 |
| FF‖MM | 0.56 (0.21, 0.86) | |
| MM‖MM | 0.55 (0.25, 0.83) | |
| Intrasexual littermate overlap: females | ||
| FFMM | 0.75 (0.18, 0.97) | F2,36 = 0.05, P = 0.95 |
| FF‖MM | 0.79 (0.24, 0.98) | |
| FF‖FF | 0.83 (0.33, 0.98) | |
| Intersexual overlap | ||
| FFMM | 0.85 (0.72, 0.94) | F1,108 = 3.23, P = 0.07 |
| FF‖MM | 0.70 (0.57, 0.81) |
Treatments are 2-sexes and all-kin group (FFMM), 2-sexes and mixed-kin group (FF‖MM), and the 1-sex groups (FF‖FF and MM‖MM). See text for details on the statistics. The body mass of a male paired with 2 females was larger than the body mass of a single male by an average of 3.64 g (±0.83 SE) (paired t test, t = 4.41, P = 0.004, n = 7).
Estimates of intrasexual littermate overlap frequencies and intersexual overlap frequencies in each treatment group and the F statistics of the differences between treatment groups
| Category . | Estimates and 95% CI . | F statistics . |
|---|---|---|
| Intrasexual littermate overlap: males | ||
| FFMM | 0.63 (0.25, 0.90) | F2,39 = 0.08, P = 0.92 |
| FF‖MM | 0.56 (0.21, 0.86) | |
| MM‖MM | 0.55 (0.25, 0.83) | |
| Intrasexual littermate overlap: females | ||
| FFMM | 0.75 (0.18, 0.97) | F2,36 = 0.05, P = 0.95 |
| FF‖MM | 0.79 (0.24, 0.98) | |
| FF‖FF | 0.83 (0.33, 0.98) | |
| Intersexual overlap | ||
| FFMM | 0.85 (0.72, 0.94) | F1,108 = 3.23, P = 0.07 |
| FF‖MM | 0.70 (0.57, 0.81) |
| Category . | Estimates and 95% CI . | F statistics . |
|---|---|---|
| Intrasexual littermate overlap: males | ||
| FFMM | 0.63 (0.25, 0.90) | F2,39 = 0.08, P = 0.92 |
| FF‖MM | 0.56 (0.21, 0.86) | |
| MM‖MM | 0.55 (0.25, 0.83) | |
| Intrasexual littermate overlap: females | ||
| FFMM | 0.75 (0.18, 0.97) | F2,36 = 0.05, P = 0.95 |
| FF‖MM | 0.79 (0.24, 0.98) | |
| FF‖FF | 0.83 (0.33, 0.98) | |
| Intersexual overlap | ||
| FFMM | 0.85 (0.72, 0.94) | F1,108 = 3.23, P = 0.07 |
| FF‖MM | 0.70 (0.57, 0.81) |
Treatments are 2-sexes and all-kin group (FFMM), 2-sexes and mixed-kin group (FF‖MM), and the 1-sex groups (FF‖FF and MM‖MM). See text for details on the statistics. The body mass of a male paired with 2 females was larger than the body mass of a single male by an average of 3.64 g (±0.83 SE) (paired t test, t = 4.41, P = 0.004, n = 7).
The selected model describing maturation probability involved the significant effects of sex (F1,163 = 9.59, P = 0.002) and season (F1,163 = 6.18, P = 0.01). The proportion of sexually mature individuals was larger in males than in females (logit contrast = 1.05, SE = 0.03) and decreased throughout the study (logit slope = −0.20 per trial, SE = 0.08). Sexual maturation was not affected by treatments when accounting for variability among replicates within each treatment (treatment: F2,56 = 2.29, P = 0.11; treatment × sex: F2,161 = 1.46, P = 0.23). However, there was a trend for delayed sexual maturation in females from the all-kin groups (Table 3). Computer simulations of maturation data assuming a binomial distribution within each sex and treatment class and no extra variation among replicates indicated that this delay was consistent (mean logit contrast = −0.964, central 95% interval = [−2.52, −0.60]). This means that our statistical power to detect differences in sexual maturation between treatments was low due to uncontrolled variations among replicates.
Number of mature males and mature females at the end of the experiment (average age = 32 days)
| Treatment groups . | Females . | Males . |
|---|---|---|
| FFMM | 10 (27) | 19 (27) |
| FF‖MM | 20 (29) | 23 (28) |
| FF‖FF and MM‖MM | 40 (57) | 42 (56) |
| Treatment groups . | Females . | Males . |
|---|---|---|
| FFMM | 10 (27) | 19 (27) |
| FF‖MM | 20 (29) | 23 (28) |
| FF‖FF and MM‖MM | 40 (57) | 42 (56) |
A chi-square test indicates that females delayed maturation in the presence of their brothers (χ2 = 9.35, df = 2, P = 0.009). Raw data are given for each treatment group. Sample size is given in parentheses.
Number of mature males and mature females at the end of the experiment (average age = 32 days)
| Treatment groups . | Females . | Males . |
|---|---|---|
| FFMM | 10 (27) | 19 (27) |
| FF‖MM | 20 (29) | 23 (28) |
| FF‖FF and MM‖MM | 40 (57) | 42 (56) |
| Treatment groups . | Females . | Males . |
|---|---|---|
| FFMM | 10 (27) | 19 (27) |
| FF‖MM | 20 (29) | 23 (28) |
| FF‖FF and MM‖MM | 40 (57) | 42 (56) |
A chi-square test indicates that females delayed maturation in the presence of their brothers (χ2 = 9.35, df = 2, P = 0.009). Raw data are given for each treatment group. Sample size is given in parentheses.
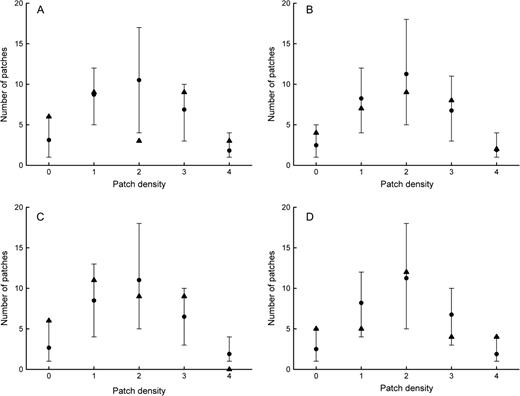
Spatial aggregation
The observed distribution of vole numbers between patches was not different from random in the FF‖MM and FF‖FF groups (Figure 3). In the MM‖MM groups, there was a deficit of patches occupied by 4 voles. In the FFMM groups, there was a deficit of patches occupied by 2 voles. When analyzing aggregation separately for males and females irrespective of treatments, similar tests showed that patches occupied by 4 males were rarely observed and that patches occupied by no females were frequently observed, compared with the expectations of a random distribution.
Spatial aggregation in the 4 treatment groups: 2-sex and all-kin group (A), 2-sex and mixed-kin group (B), male mixed-kin group (C), and female mixed-kin group (D). Mean circles and central 95% interval of the number of patches inhabited by 0, 1, 2, 3, and 4 voles according to the Monte Carlo simulation of a random distribution. The observed values are represented with triangles.
DISCUSSION
We manipulated the presence of opposite-sex littermates and the juvenile sex ratio to tease apart the effects of competition, cooperation, and inbreeding avoidance on space use and sexual maturation in juvenile root voles. We assessed space use using capture location rather than more accurate home range measurements. In a similar patchy habitat, a previous study found that as high as 85% of the animals captured in one patch had a territory located in the same patch (Gundersen and Andreassen 1998). Hence, our data were sufficient to ascertain the home range of an individual. We found that intersexual segregation was stronger when opposite-sex root voles were unrelated, which was due to a male-biased natal dispersal in the 2-sex and mixed-kin group. Furthermore, natal dispersal was unrelated to the juvenile sex ratio, females tended to aggregate spatially, and there was a consistent overlap among sisters. Thus, our results do not support the classical model of a polygynous mating system where natal dispersal should be flexibly adjusted to reduce the risks of intrasexual competition and inbreeding (Greenwood 1980; Perrin and Mazalov 2000). This conclusion is strengthened by previous observations from nonmanipulated litters, in which young males did not seem to avoid their sisters, whereas young females clustered intrasexually (Gundersen and Andreassen 1998).
Experimental design
Our experimental design only represents one form of patchiness in terms of patch size and interpatch distances. However, root voles of the geographical strain we have studied typically inhabit highly fragmented habitats as the one used here (Tast 1966). Furthermore, interpatch distance (26 m) was sufficiently large to inhibit movements of established animals between patches. Female root voles generally avoid crossing areas of open habitats when interpatch distances are larger than 5–7 m (Ims et al. 1993). Earlier radiotelemetry studies have shown that as few as 10% of recorded movements occurred between vegetation fragments separated by 7–15 m of open habitats (Andreassen and Ims 1998). In males, gaps as small as 4 m cause a 5-fold decrease in movement rates (Andreassen et al. 1996). These results indicate that the interpatch movements observed here were true dispersal events rather than within–home range relocations.
The duration of our field trials was short, but the experiments were conducted during the most critical stage for sexual maturation, natal dispersal, and settlement during the summer season (Bondrup-Nielsen and Karlsson 1985; Andreassen and Ims 2001). Thus, the duration of our trials was enough to draw conclusions about space use and settlement. Our experiment did not involve reproductive adults (either parents or others). In the absence of adult males, puberty can be delayed and philopatry can be facilitated, especially in females (Wolff 1994). However, releasing offspring in the presence of their parents would have biased our manipulation because the presence of opposite-sex siblings would have been confounded with the presence of parents, which is known to influence dispersal (Wolff 1994). Hence, we consider that the social setting of our experiment was the best choice to investigate siblings' interactions and natal dispersal in root voles.
Intrasexual competition
We found no evidence that young root voles dispersed more frequently when they competed for resources with individuals from the same sex than when they competed for equivalent resources with individuals from the opposite sex. As the juvenile sex ratio manipulation changed mate availability, which was lower for males (respectively, females) in male-biased (respectively, female-biased) populations, our results also suggest that mate availability did not influence natal dispersal. Furthermore, the absence of body growth and survival differences between treatments—2 traits that are influenced by competition for food and space in this species (Gundersen et al. 2002)—indicates that sexual asymmetries in competition were weak or not existent. Thus, juveniles might share similar ecological requirements irrespective of their sex, and the initial settlement of juveniles might depend on the spatial distribution of food resources in both sexes. This could explain an apparent contradiction with previous observations on adult settlement patterns, where dispersal and space use were primarily influenced by competition for food in adult females and by competition for mates in adult males (Ostfeld 1985; Ims 1987). Future studies should try to better understand the ontogeny of sexual differences in critical resource requirements and associated changes in dispersal patterns.
However, we found that females tended to aggregate in space, that patches occupied by 4 males were less frequent than expected at random, and that larger males associated preferentially with females compared with smaller males. This sex-specific distribution pattern is consistent with the spacing system envisioned for Microtus species, where adult males defend territories with several females, whereas females within the male territory share space to a greater extent (Ostfeld 1985; Ims 1987). Previous studies also showed that male root voles are more territorial and defend larger home ranges than females (Andreassen et al. 1998; Gundersen and Andreassen 1998).
Inbreeding and kin competition avoidance
Inbred mating among siblings may be avoided by a variety of mechanisms, such as delayed maturation in juveniles from siblings groups, mating preferences for nonsiblings, multiple mating, or dispersal behavior. Avoidance of siblings through natal dispersal has been found under experimental conditions in the meadow vole (Bollinger et al. 1993), in the female red-backed voles (Kawata 1987), and in the common lizard (Le Galliard et al. 2003). However, in male gray-sided voles, male natal dispersal was not influenced by the presence of siblings (Ims and Andreassen 1991). Here, neither did opposite-sex littermates trigger natal dispersal nor did natal dispersal result in spatial segregation among opposite-sex littermates, suggesting that inbreeding avoidance did not affect natal dispersal. Furthermore, same-sex littermates did not avoid overlapping in space. Thus, natal dispersal was also apparently not sensitive to competition among same-sex kin.
One explanation for the absence of spatial segregation among opposite-sex siblings might be that root voles can avoid mating with relatives using alternative mechanisms for kin discrimination (e.g., delayed maturation or mating preferences) and thus retain the benefits of outbreeding without paying the costs of dispersal (Perrin and Goudet 2001). In accordance with this explanation, we found that female root voles tended to delay maturation in the presence of opposite-sex littermates, suggesting that inbreeding avoidance could have induced delayed breeding in females. Another possibility is that inbreeding depression is too weak to select for inbreeding avoidance by natal dispersal in this species. In root voles originating from the same geographic area as in our study, full-sib mating led to reduced reproductive performances in the laboratory, but the inbreeding depression was only pronounced after 3 generations of inbred mating (dos Santos et al. 1995) and was not significant under field conditions (Gundersen et al. 2001). Lehmann and Perrin (2003) modeled kin discrimination and dispersal and found that the evolution of active inbreeding avoidance by kin recognition required strong inbreeding loads, equivalent to a one-third reduction in offspring fitness in the case of full-sib mating. Future theoretical studies should investigate more thoroughly the interactions among kin recognition mechanisms, delayed maturation, and natal dispersal.
Kin clusters in females
Males dispersed more than females in the 2-sex and mixed-kin groups, opposite-sex littermates shared space more frequently than opposite-sex unrelated voles, and female siblings clustered in space. Female kin clusters were caused by female philopatry because kin clusters occurred in the release patch but not in the immigration patch. Altogether, these results are consistent with the kin cooperation hypothesis, which states that cooperative interactions among resident females increase the benefits of philopatry and favor the buildup of female kin structures in the natal habitat (Solomon 2003). The similarity between the kin clustering observed here and previous observations of space use in female root voles (Andreassen et al. 1998; Bjørnstad et al. 1998; Gundersen and Andreassen 1998) strengthens the view that kin clusters of philopatric females are a basic component of the social structure in the root vole.
Space sharing among relatives is common in small mammals and is often associated with elevated population density when high-quality territories are filled and individuals are forced to refrain dispersal from their natal area or when resources are aggregated in space (McGuire et al. 1993; Solomon 2003). There are, however, also potential social benefits associated with space sharing, such as an improved protection against predators or defense against infanticide performed by unfamiliar animals (Mappes et al. 1995; Lambin and Yoccoz 1998). Our observations lend supports to the latter explanation as individuals did not distribute themselves as to maximize the territory size, but rather, contra the predictions of a resource competition hypothesis, females tended to aggregate in space and did not avoid competition with nonsibling females by dispersing more in mixed-kin groups. Thus, we suggest that space sharing among sisters might be a tactic to defend the natal home range against unfamiliar males. The formation of female kin clusters along with increased female aggressiveness against unfamiliar males may have caused increased dispersal of unrelated males and compelled unrelated males to avoid females.
For many rodents, infanticide seems to occur frequently enough to contribute significantly to juvenile mortality and thus has the potential to be a strong evolutionary force regarding the development of defensive tactics (Agrell et al. 1998). Improved reproductive success through cooperative defense against infanticidal conspecifics has been reported in house mice, Belding's ground squirrels, several social carnivores, and some primate species (Sherman 1980; Manning et al. 1995; Ebensperger 1998). Female rodents often use aggression to keep males out from their nest sites (Wolff 1985), and larger female body size is correlated with improved defense intensity and higher weaning success (Jonsson et al. 2002). Breeding male root voles are approximately 30% larger than females, and thus, the potential advantage of cooperation among breeding females should be large. Recently, manipulations of the population turnover of male root voles found that the presence of unfamiliar males strongly reduces survival and weaning success in resident females (Andreassen and Gundersen 2006). These results indicate that aggression is a common tactic used by male root voles. In light of our results, future experiments should try to evaluate the joint influences of spatial association between littermates and infanticide risks on the reproductive success of young females.
CONCLUSION
Our experiments found no evidence that natal dispersal was flexibly adjusted to reduce the risks of intrasexual competition and inbreeding among sibling root voles. Rather, sister root voles clustered in the release patch and deterred unfamiliar males from sharing space with them. This result might be explained by resource defense in females against the threat of aggressive or infanticidal unfamiliar males. The ensuing increase in risks of inbred mating among littermates was partially avoided because females tended to delay sexual maturation in the presence of their brothers. Understanding the mechanisms underlying the development of kin structures among females is important because kin cooperation may feed back on population dynamics and on the evolution of complex social behaviors.
We thank 2 anonymous reviewers, as well as T. Ergon and R.A. Ims, for thorough and helpful comments on a previous version of the manuscript. We acknowledge the kind help of K. Hoset and L. Korslund who provided the breeding pairs used in our experiment. We thank F. Dufour and D. Mersch for assistance in the field and the personnel at the Animal Division in Oslo for help in the laboratory. This project was funded by a grant from the Sixth Framework Programme of the European Commission (Marie Curie Intra-European Fellowship FP6-501658) to J.-F.L.G.
References
Agrell J, Wolff JO, Ylonen H.
Andreassen HP, Gundersen G.
Andreassen HP, Hertzberg K, Ims RA.
Andreassen HP, Ims RA.
Andreassen HP, Ims RA.
Andreassen HP, Ims RA, Steinset OK.
Bjørnstad ON, Andreassen HP, Ims RA.
Bollinger EK, Harper SJ, Barrett GW.
Bondrup-Nielsen S, Ims RA.
Bondrup-Nielsen S, Karlsson S.
Boonstra R, Krebs CJ, Gaines MS, Johnson ML, Craine ITM.
Clobert J, Ims RA, Rousset F.
Dobson FS.
dos Santos EM, Andreassen HP, Ims RA.
Ebensperger LA.
Emlen ST, Oring LW.
Greenwood PJ.
Gundersen G, Aars J, Andreassen HP, Ims RA.
Gundersen G, Andreassen HP.
Gundersen G, Andreassen HP, Ims RA.
Hovland N, Andreassen HP, Ims RA.
Ims RA, Andreassen HP.
Ims RA, Rolstad J, Wegge P.
Jonsson P, Agrell J, Koskela E, Mappes T.
Kawata M.
Lambin X.
Lambin X.
Lambin X, Aars J, Piertney SB.
Lambin X, Krebs CJ, Scott B.
Lambin X, Yoccoz NG.
Le Galliard J-F, Ferrière R, Clobert J.
Lehmann L, Perrin N.
Littell RC, Millinken GA, Stroup WW, Wolfinger RD.
Manning CJ, Dewsbury DA, Wakeland EK, Potts WK.
Mappes T, Ylönen H, Viitala J.
McGuire B, Getz LL, Hofmann JE, Pizzuto T, Frase B.
Paz y Miño G, Leonard ST, Ferkin MH, Trimble JF.
Perrin N, Goudet J.
Perrin N, Lehmann L.
Perrin N, Mazalov V.
Pusey AE.
Sherman PW.
Shields WM.
Solomon NG.
Stamps JA.
Stenseth NC, Lidicker WZ.
Tast J.
Wolff JO.
Author notes
aCentre for Ecological and Evolutionary Synthesis, University of Oslo, PO Box 1050, Blindern, Oslo NO-0316, Norway
bFaculty of Forestry and Wildlife Management, Hedmark University College, Koppang NO-2480, Norway