-
PDF
- Split View
-
Views
-
Cite
Cite
Dottie M. Clower, Richard P. Dum, Peter L. Strick, Basal Ganglia and Cerebellar Inputs to ‘AIP’, Cerebral Cortex, Volume 15, Issue 7, July 2005, Pages 913–920, https://doi.org/10.1093/cercor/bhh190
Close - Share Icon Share
Abstract
The anterior intraparietal area (AIP) is a subregion of area 7b in posterior parietal cortex. AIP neurons respond to the sight of objects, as well as to the act of grasping them. We used retrograde transneuronal transport of rabies virus to examine subcortical inputs to AIP in the monkey. Virus transport labeled substantial numbers of neurons in the substantia nigra pars reticulata (SNpr), as well as in the dentate nucleus of the cerebellum. The hotspots of labeled neurons in SNpr and in dentate after AIP injections were separate from those created by virus injections into several other parietal or frontal regions. These observations provide the first evidence that a major output nucleus of the basal ganglia, the SNpr, projects to a region of posterior parietal cortex. In addition, our findings provide further support for the concept that posterior parietal cortex is a target of cerebellar output.
Introduction
Physiological and anatomical studies in monkeys have identified a unique subregion of area 7b within inferior parietal cortex termed the anterior intraparietal area (AIP). AIP neurons either have visual responses to the three-dimensional features of objects, motor responses to object manipulation or the combination of the two types of responses (Godschalk and Lemon, 1989; Taira et al., 1990; Sakata and Taira, 1994; Sakata et al., 1995, 1998; Murata et al., 1996, 2000). AIP can be defined anatomically based on its extensive interconnections with the ventral premotor area (PMv or area F5) (Chavis and Pandya, 1976; Petrides and Pandya, 1984; Godschalk et al., 1984; Matelli et al., 1984, 1986; Neal et al., 1990; Ghosh and Gattera, 1995; Luppino et al., 1999; Tanne-Gariepy et al., 2002; see also Preuss and Goldman-Rakic, 1991; Lewis and Van Essen, 2000). Indeed, neurons in the PMv display visuomotor responses that are in many respects similar to those of AIP neurons. However, AIP contains more neurons that are exclusively responsive to the visual features of an object, whereas PMv contains more neurons that are selectively responsive during movement (Murata et al., 1997, 2000). Thus, AIP and PMv are thought to be nodes in a cortical network concerned with processing the visual features of objects to specify the appropriate grasping patterns for manipulating them (Sakata et al., 1997; Oztop and Arbib, 2002).
With the exception of some cortical interconnections, the anatomical inputs to AIP have not been well characterized. In recent studies we have shown that an adjacent portion of area 7 is the target of output from the dentate nucleus of the cerebellum while other portions of area 7 receive input from either the superior colliculus or CA1 of the hippocampus (Clower et al., 2001a). We wondered whether one or all of these subcortical systems were a source of input to AIP. For example, perhaps some of the ‘visual’ properties of AIP neurons are the result of input from the superior colliculus. Similarly, some of the ‘motor’ properties of AIP neurons could be a consequence of input from the cerebellum. In addition, given the extensive interconnections between AIP and PMv, we sought to examine whether these two cortical areas received inputs from common sources. PMv is the target of output from both the dentate and the internal segment of the globus pallidus (GPi) (Hoover and Strick, 1993; Dum and Strick, 2002). While there is no prior evidence for basal ganglia input to regions of posterior parietal cortex, some basal ganglia disorders result in symptoms that closely mimic parietal lobe dysfunction (Danta and Hilton, 1975; Boller et al., 1984; Richards et al., 1993; Hocherman and Giladi, 1998; Barrett et al., 2001; Lee et al., 2001a,b; Montse et al., 2001). These observations raise the possibility that regions of posterior parietal cortex might also be targets of output from the basal ganglia.
To examine these issues, we used retrograde transneuronal transport of rabies virus after localized injections into AIP. The parameters for using rabies as a transneuronal tracer have been examined extensively in the cebus monkey (e.g. Kelly and Strick, 2000, 2003). In addition, we have examined the subcortical inputs to a number of cortical regions in the cebus monkey, including areas of posterior parietal, motor, prefrontal and inferotemporal cortex (Hoover and Strick, 1993, 1999; Lynch et al., 1994; Middleton and Strick, 1996, 1997, 2001; Clower et al., 2001a,b; Middleton and Strick, 2001; Dum and Strick, 2003). To be able to use all of this prior data for comparison, we chose to perform this experiment in the same species. However, prior anatomical and physiological experiments on AIP have been performed in macaques. Because AIP can be defined based on its interconnections with PMv, we used this anatomical feature to identify the location of AIP in the cebus monkey. We then injected the cebus AIP with rabies virus to define its subcortical inputs.
Our results show that AIP is the target of both basal ganglia and cerebellar output. Approximately equivalent numbers of neurons from each subcortical system project to AIP. In addition, the number of basal ganglia and cerebellar neurons that project to AIP is comparable to the number that project to individual areas of premotor and prefrontal cortex. Furthermore, the origins of the densest subcortical inputs to AIP are largely separate from the origins of the densest input to PMv. Abstracts of some of these results have been reported elsewhere (Clower et al., 2001b, 2002).
Materials and Methods
The procedures adopted for this study and the care provided experimental animals conformed to the regulations detailed in the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All protocols were reviewed and approved by the Institutional Animal Care and Use Committees. The biosafety precautions taken during rabies virus tracer experiments conformed to or exceeded Biosafety Level 2 (BSL-2) regulations detailed in Biosafety in Microbiological and Biomedical Laboratories (Health and Human Services Publication 93-8395). A detailed description of the procedures for handling virus and virus-infected animals is presented in Kelly and Strick (2000); see also Strick and Card (1992) and Hoover and Strick (1999).
To conduct these experiments it was necessary to identify the location of AIP in the cebus monkey. In macaques, AIP is the region of posterior parietal cortex that is most densely connected with the PMv. We used this connection to define AIP in the cebus monkey. In two monkeys we located the region of area 7 that contained large numbers of labeled neurons after an injection of a conventional tracer into the digit representation of the PMv.
In three monkeys we injected AIP with rabies virus to label basal ganglia and cerebellar neurons that project (via the thalamus) to this cortical area. Finally, in an additional animal, we injected the AIP with conventional tracers to identify the source of its thalamic input.
Conventional Tracer Experiments
Surgical Procedures
Our methods for intracortical stimulation and tracer injections have been described fully in prior publications (Strick and Preston, 1982a,b; Holsapple et al., 1991; Hoover and Strick, 1999). Briefly, all surgical procedures were performed under aseptic conditions. Once the animal was anesthetized with Telazol, it was placed in a stereotaxic frame and an appliance was secured to its skull with small screws and dental acrylic. The appliance served as an atraumatic anchor to hold the animal's head during the stimulation phase of the experiment. A large craniotomy was performed over the appropriate cortical area and the dura was opened. The cortical surface was protected from desiccation with warm surgical grade silicone.
Physiological Mapping
We used parylene coated Elgiloy microelectrodes (Suzuki and Azuma, 1976; impedance = 0.6–1.4 MΩ @ 1 kHz) to deliver intracortical stimuli. Cathodal pulses (12–32 pulses, 0.2 ms duration, 333 Hz, 1–50 μA) from a constant current stimulator were delivered at a standard depth of 1500 μm. The motor response evoked by intracortical stimulation was determined by visual observation and muscle palpation. The response at each site was defined as the movement or muscle contraction that was evoked at threshold- the stimulus intensity at which the response occurred on 50% of the trials. The results of physiological mapping were entered into a custom designed computer database for on-line acquisition and analysis of data from mapping experiments.
Tracer Injections
After we identified the digit representations in the PMv, we used a Hamilton syringe with a 28 gauge fixed needle to inject diamidino yellow (DY, 2% in distilled water, 0.4 μl per injection) into the PMv of one animal (R15) and fast blue (FB, 5% in distilled water, 0.3 μl per injection) into the PMv of the other (R24). Penetrations were spaced ∼1 mm apart except to avoid blood vessels. The tracers were injected at a depth of 1.5 mm below the cortical surface. The needle was allowed to remain in place for 3 min after each injection. When the injections were completed, the exposed cortical surface was covered with dura and silastic, and the wound closed in anatomical layers. After a 12 day survival period, each animal was tranquilized (ketamine, 25 mg/kg, i.m.), deeply anesthetized (Nembutal, 36 mg/kg, i.p.) and perfused transcardially using a multi-stage procedure (Rosene and Mesulam, 1978; Mesulam, 1982).
Tissue Processing
Following the perfusion, the brain was blocked and stored at 4°C with 20% glycerin added as a cryoprotective agent for 4–7 days (Rosene et al., 1986). Blocks of brain were frozen (Rosene et al., 1986) and serially sectioned in the coronal plane at a thickness of 50 μm. Every tenth section was counterstained with cresyl violet for cytoarchitectonic analysis (Mesulam, 1982).
Analytic Procedures
Every fourth to eighth section was examined for DY and FB labeled neurons using a light microscope and epifluorescence illumination (Leitz filter D). Injection sites, section outlines and labeled cells were plotted using a computer-based charting system (MD2, Minnesota Datametrics). This system uses optical encoders to sense X–Y movements of the microscope stage and stores the coordinates of charted structures (e.g. section outlines, injection site zones and labeled neurons). The extent of the injection site was compared with the location of the identified digit representation by superimposing the outline of the reconstructed injection site over the map of movements evoked at each penetration site. Maps of individual sections through parietal cortex were aligned interactively on the computer. Sections were unfolded and used to construct 2-D maps of the cortex that indicated the location of labeled neurons in relation to morphological features (Dum and Strick, 1991; He et al., 1993, 1995).
Conventional Tracer Injection into AIP
Six injections of DY (2% in distilled water, 0.4 μl per injection) were placed in AIP. The injections were made ∼1.5 mm apart at a depth of 1.5 mm. Sections were plotted every 200 μm through the thalamus. All other procedures were as described above.
Virus Tracer Experiments
Virus Injections
We injected rabies virus into the AIP region of parietal cortex of three monkeys (Cebus apella). The method for rabies virus surgery has been described previously (Kelly and Strick, 2000). Briefly, the animals were anesthetized with isoflurane, and a craniotomy was performed over the parietal lobe. The dura was incised and reflected to expose the cortex. We used a 5 μl Hamilton syringe with a 28–32 gauge removable needle to place multiple injections (0.2 μl per site) of virus into the targeted region. Injections were spaced 0.5–1.0 mm apart except to avoid blood vessels. Ninety injections were made in each animal (three injection depths at 30 injection sites). Following each injection into the cortex, the needle was left in place for 2 min. Surgery was completed as described above. Following the surgery, animals were placed in a BSL-2 isolation room for further observation and recovery. All animals received dexamethasone (0.1–0.5 mg/kg, i.m. or p.o.) and buprenorphine (Buprenex®, 0.01 mg/kg, i.m.). After a survival period of 3–4 days, the animals were perfused as described previously.
Tissue Processing
Brain tissue was processed as described in the previous section. To identify neurons labeled by virus transport, we processed free-floating tissue sections according to the avidin-biotin-peroxidase method (ABC®; Vectastain, Vector Laboratories, Inc., Burlingame, CA) using antibodies directed against rabies virus (Kelly and Strick, 2000). At least every other section from these animals was reacted. Sections were mounted onto gelatin-coated glass slides, air dried, and then coverslipped with either Artmount® or DPX®.
Analytic Procedures
Approximately every other section was examined for labeled neurons under bright-field, dark-field, and polarized illumination. Sections through the injection sites, frontal lobe, GPi, SNpr and dentate nucleus were plotted using a computerized charting system (MD2, Minnesota Datametrics, Inc., St. Paul, MN).
Results
Location of AIP in the Cebus Monkey
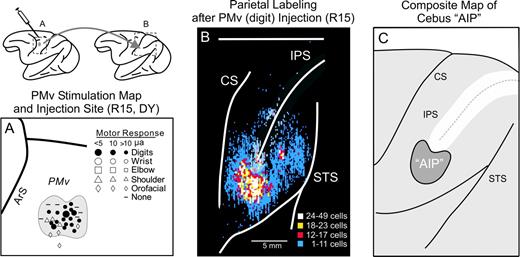
AIP is the region of posterior parietal cortex that is most densely interconnected with the PMv (Luppino et al., 1999; Tanne-Gariepy et al., 2002). Therefore, to identify AIP in the cebus monkey we examined the origin of parietal lobe projections to the hand representation of the PMv in two animals. We used intracortical stimulation to define the hand representation in each animal prior to the tracer injections (Fig. 1A). We then injected the hand area with a fluorescent tracer. In animal R15 we injected DY and in animal R24 we injected FB.
Localization of AIP in the cebus monkey based on corticocortical connectivity with PMv. (A) Map of microstimulation sites (symbols) on the cortical surface of cebus monkey R15 and boundary of the digit region of PMv injected with diamidino yellow (DY). (B) Cortical surface map showing density and location of labeled neurons after DY injection at the site shown in (A). (C) Composite map indicating the region within parietal cortex where densest labeling occurred after injection of the digit representation in PMv in experiments R15 and R24 (shaded area). AIP, anterior intraparietal area; ArS, arcuate sulcus; CS, central sulcus; IPS, intraparietal sulcus; PMv, ventral premotor area; STS, superior temporal sulcus.
In both animals we found large numbers of labeled neurons on the cortical surface just lateral to the tip of the intraparietal sulcus (Fig. 1B,C). The regions of dense labeling in the two animals overlapped considerably. The labeled region extended rostral to the tip of the intraparietal sulcus in R15 and caudal to the tip of the sulcus in R24. We operationally defined AIP as the region where dense labeling was present in both animals (Fig. 1C, ‘AIP’). All of our virus injection sites were aimed at this region. Note that AIP in the cebus monkey is somewhat lateral and rostral to the location of AIP in the macaque (Godschalk et al., 1984; Matelli et al., 1984, 1986; Taira et al., 1990; Ghosh and Gattera, 1995; Murata et al., 2000; Tanne-Gariepy et al., 2002).
Subcortical Input to AIP
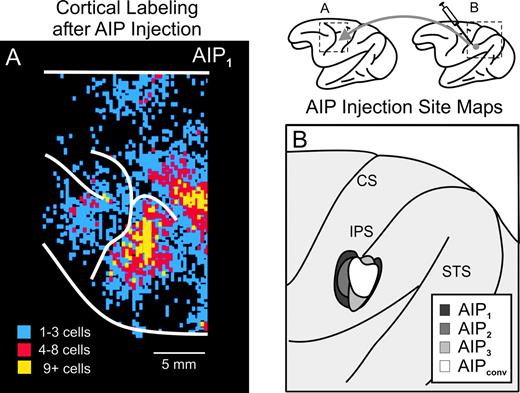
We injected AIP with rabies virus (CVS-11) in three cebus monkeys (Fig. 2B). We set the survival time (3–4 days) to allow retrograde transport of virus from the injection site to first-order neurons in the thalamus and then, retrograde transneuronal transport of virus from these first-order neurons to second-order neurons in subcortical sites. Maps of labeled neurons in the frontal lobe showed that large numbers of labeled neurons overlapped the hand representation of the PMv in each animal (e.g. Fig. 2A). This observation confirmed that the injection sites were accurately placed within AIP.
Localization of injection sites in AIP of the cebus monkey. (A) Cortical surface map showing the density and location of labeled neurons in the hand region of PMv after injections of rabies in AIP1. (B) Composite map of AIP injection sites for three rabies experiments (AIP1–AIP3) and one conventional tracer (DY) experiment (AIPconv).
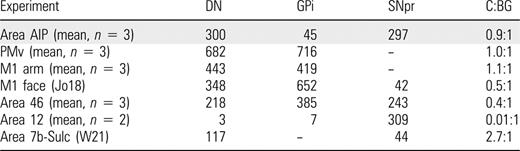
We scanned every fifth section through basal ganglia, cerebellum, hippocampus and superior colliculus for second-order neurons labeled by transneuronal transport of virus. Substantial numbers of labeled neurons were found only at two sites: the substantia nigra (mean = 297, range = 180–490) and the dentate nucleus (mean = 283, range = 152–526) (Figs 3 and 4, Tables 1 and 2). A small number of labeled neurons also were consistently found in GPi (mean = 45, range = 24–64). A moderate number of labeled neurons (n = 94) were present in the posterior interpositus in only one (AIP2) of the three animals. In total, the number of labeled neurons in the output nuclei of the cerebellum and basal ganglia varied from animal to animal by a factor of ∼3 (Table 1). However, the ratio of cerebellar to basal ganglia output neurons was nearly 1:1 in all three animals. Thus, AIP is the target of output from both the cerebellum and the basal ganglia, and the magnitude of this output is comparable from the two sources.
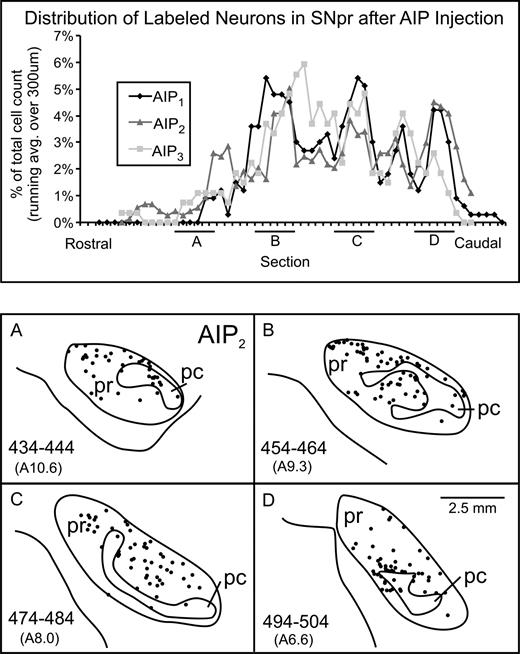
Distribution and density of SNpr neurons that project to AIP. Top: rostrocaudal distribution of labeled neurons in SNpr after AIP injections of rabies in three experiments (AIP1–AIP3). Bottom: cross sections of the substantia nigra showing the location of ‘second-order’ neurons (dots) labeled after transport of rabies virus injected in AIP. Each panel includes the labeled neurons from five sections (100 μm apart). Numbers in parentheses below section numbers indicate approximate rostrocaudal position of the sections according to Olszewski (1952). SNpc; substantia nigra pars compacta; SNpr, substantia nigra pars reticulata.
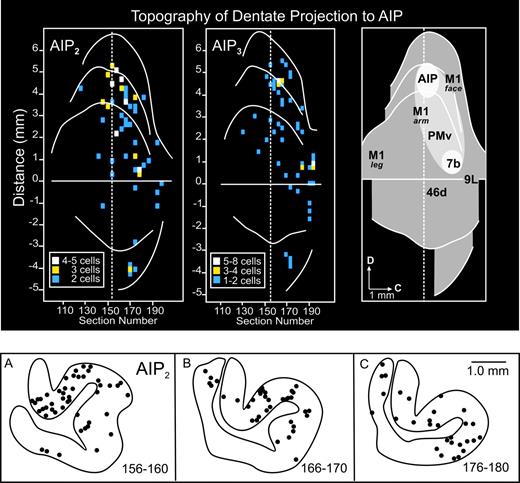
Distribution and density of dentate neurons that project to AIP. Top: unfolded map of the dentate nucleus of AIP2 (left), AIP3 (middle), and composite map (right). The maps of the dentate were created by unfolding serial coronal sections through the nucleus (for detailed methods, see Dum and Strick, 2003). The map was reconstructed from every other coronal section through the nucleus. White regions on the composite map indicate areas of densest labeling. The light gray region indicates the location of the low-density field of labeled neurons (see text for details). The labels on the composite map indicate the location of dentate output channels to other frontal, motor and parietal areas of cortex. Bottom: cross sections of the dentate nucleus showing the location of ‘second-order’ neurons (dots) labeled after transport of rabies virus from AIP. Each panel includes the labeled neurons from three sections (100 μm apart).
Subcortical labeling after area AIP injections
| Experiment . | DN . | GPi . | SNpr . | C:BG . |
|---|---|---|---|---|
| AIP1 (DC1) | 202 | 64 | 222 | 0.7:1 |
| AIP2 (DC4) | 526 | 48 | 490 | 1.0:1 |
| AIP3 (DC6) | 172 | 24 | 180 | 0.8:1 |
| Experiment . | DN . | GPi . | SNpr . | C:BG . |
|---|---|---|---|---|
| AIP1 (DC1) | 202 | 64 | 222 | 0.7:1 |
| AIP2 (DC4) | 526 | 48 | 490 | 1.0:1 |
| AIP3 (DC6) | 172 | 24 | 180 | 0.8:1 |
Subcortical labeling after area AIP injections
| Experiment . | DN . | GPi . | SNpr . | C:BG . |
|---|---|---|---|---|
| AIP1 (DC1) | 202 | 64 | 222 | 0.7:1 |
| AIP2 (DC4) | 526 | 48 | 490 | 1.0:1 |
| AIP3 (DC6) | 172 | 24 | 180 | 0.8:1 |
| Experiment . | DN . | GPi . | SNpr . | C:BG . |
|---|---|---|---|---|
| AIP1 (DC1) | 202 | 64 | 222 | 0.7:1 |
| AIP2 (DC4) | 526 | 48 | 490 | 1.0:1 |
| AIP3 (DC6) | 172 | 24 | 180 | 0.8:1 |
In the substantia nigra, labeled neurons were broadly distributed in the caudal two-thirds of the nucleus within the pars reticulata (SNpr) (Fig. 3). Although the absolute number of neurons labeled in the nigra varied from animal to animal (Table 1), the rostro-caudal distribution of labeled neurons was relatively consistent (Fig. 3, top). At rostral levels of the nigra, second-order neurons tended to be located in the dorsal portion of the nucleus. The distribution of labeled neurons shifted to a more ventral location at more caudal levels of the nucleus. Some authors have further subdivided the nigra into a pars reticulata region where cells uniformly do not stain with tyrosine hydroxylase (TH-negative) and a pars mixta region where TH-negative and TH-positive cells are intermingled (Francois et al., 1984; Langer and Graybiel, 1989; Williams and Goldman-Rakic, 1998). The nigra neurons labeled after virus injections into AIP were found in both of these regions.
In the dentate nucleus, labeled neurons were widely distributed in the caudal two-thirds of the nucleus (Fig. 4). In all three of our experimental animals the densest site of labeled neurons after AIP injections was located dorsally at mid rostro-caudal levels of the dentate (Fig. 4, top-left, top-middle, A,B). A second small patch of dense labeling was present in two of the three animals (AIP2 & AIP3) in the caudal third of the dentate (Fig. 4, top-left, top-middle, C). These labeled neurons were located at a site we have previously shown to be near the origin of output to an adjacent portion of area 7b (Fig. 4, top-right; Clower et al., 2001a,b; Dum et al., 2002; Dum and Strick, 2003). A third, very small patch of labeled neurons was present in two of the three animals (AIP2 & AIP3) in the most ventral and medial part of the dentate (Fig. 4, top-left, top-middle, B). Finally, a low-density field of labeled neurons was present between the dorsal AIP site and the more caudal and ventral area 7b site (Fig. 4, top-left and top-right). Scattered labeled neurons were found in this region in all the animals that received virus injections into AIP. The lightly shaded ellipse in Fig. 4 (top-right) indicates an approximation of the limits of this field. Despite its low density, the field contained between two-thirds and three-quarters of the total cells that project from the dentate (via the thalamus) to AIP. It is notable that the field overlaps the region of the dentate that we have previously shown to be the origin of projections to M1 and the PMv (Fig. 4, top-right; Dum et al., 2002; Dum and Strick, 2003).
‘Direct’ Subcortical Input AIP
In one cebus monkey we used a conventional tracer to: (i) identify thalamic nuclei that might mediate the retrograde transneuronal transport from AIP to the cerebellum and basal ganglia and (ii) confirm that no cerebellar or basal ganglia neurons project directly to AIP. Injections of DY into AIP (Fig. 2B, AIPconv) resulted in focal accumulations of labeled neurons in multiple thalamic nuclei including the medial and lateral pulvinar, the oral pulvinar, LP, CnMd, VPLc, VPLo, VLc, CL, MDmf, VPI, VLm and PCn (not illustrated). Regions in several of these thalamic nuclei (e.g. VLc, MDmf, CL) are known to be the site of termination of cerebellar or nigral efferents (for references and review, see Percheron et al., 1996). Thus, the dentate and nigral labeling we observed after virus injections could be mediated by retrograde transneuronal transport via these regions of the thalamus.
DY injections into AIP labeled a small number of neurons in A9 near the dorsal border of SNpr. However, no labeled neurons were seen in the core of SNpr, GPi or in any of the deep cerebellar nuclei following these injections. Thus, all of the labeled neurons we found in the basal ganglia and cerebellum following AIP injections of virus were second-order neurons labeled by retrograde transneuronal transport via the thalamus.
Discussion
Virus tracing has enabled us to demonstrate that AIP is the target of both cerebellar and basal ganglia output. Indeed, the number of basal ganglia and cerebellar neurons that project to AIP is comparable to the number that project to individual areas of motor and prefrontal cortex (Table 2). Thus, a considerable component of basal ganglia and cerebellar output is devoted to influencing the functional operations of posterior parietal cortex. In a prior study we examined subcortical inputs to other portions of area 7. We found that area 7a is the target of output from CA1 in the hippocampus, that the lateral intraparietal area (LIP) is the target of output from the superior colliculus and that a sulcal region of area 7b is the target of cerebellar output (Clower et al., 2001a,b). These results, together with those from the present study, indicate that there is a complex topography to the pattern of subcortical inputs to different subregions of posterior parietal cortex.
Cerebellar Input to AIP
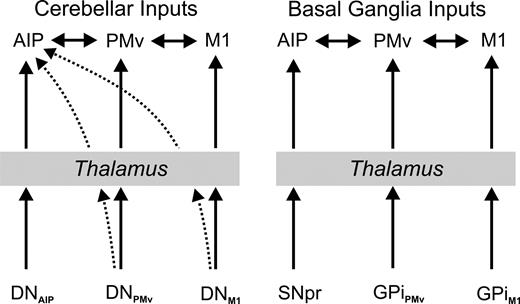
To date, we have examined the organization of cerebellar projections to regions of M1, several premotor areas, three areas of dorsolateral prefrontal cortex, a portion of the frontal eye field and a subregion within area 7b (Lynch et al., 1994; Middleton and Strick, 1994, 1996, 1997, 2000, 2001; Hoover and Strick, 1999; Clower et al., 2001a,b; Dum et al., 2002; Dum and Strick, 2003). These cortical areas each receive input from a focal cluster of dentate neurons that we termed an ‘output channel’ (for references and review, see Dum et al., 2002; Dum and Strick, 2003). In all of our prior studies, we found that the output channels projecting to different cortical areas originate from topographically separate regions of the dentate.
The results of the present study indicate that the ‘output channel’ concept does not fully characterize the pattern of cerebellar projections to AIP. AIP is unique in receiving a broadly distributed, as well as a focal projection from the dentate. The focal projection originates from a small cluster of dentate neurons that is located dorsally in the dentate at mid rostro-caudal levels. This cluster forms an output channel (via the thalamus) to AIP that is spatially separate from those that project to other cortical areas. AIP also receives input from dentate neurons that are broadly distributed within the nucleus. These neurons are located in dentate regions that also contain output channels to M1, the PMv and perhaps other premotor areas (Dum and Strick, 1999, 2003; Akkal et al., 2001). The local density within this field of labeled neurons is low. However, the entire labeled region contains a substantial component (two-thirds to three-quarters) of the dentate neurons that project to AIP.
Overall, our results indicate that AIP is the target of two patterns of input from the dentate– a small output channel and a broadly distributed field of neurons. This creates a situation in which AIP may receive a sample of the dentate output that is streaming to motor areas in the frontal lobe and may integrate this information with that from its own output channel (Fig. 5, left). The results from other anatomical and physiological studies are consistent with this interpretation (Sasaki et al., 1976; Kakei and Shinoda, 1990; Wannier et al., 1992; Kakei et al., 1995). Thus, some of the complex sensorimotor properties of AIP neurons (Godschalk and Lemon, 1989; Taira et al., 1990; Sakata and Taira, 1994; Sakata et al., 1995, 1998; Murata et al., 1996, 2000) may result from the integration of these inputs. For example, neurons in AIP and PMv have similar neural response patterns during visually guided hand manipulation tasks, and many neurons in both areas show selectivity for both the visual presentation of 3-D objects, as well as the appropriate hand movement for manipulation of a presented object (Murata et al., 1997, 2000). Imaging studies during object manipulation have found activation of regions of the human premotor and parietal cortex that may be analogous to the monkey PMv and AIP (Binkofski et al., 1999; Jancke et al., 2001; Mecklinger et al., 2002; Stoeckel et al., 2003). These and other findings have led to the proposal that PMv and AIP together provide a neural substrate for translating the visual properties of a 3-dimensional object into the appropriate hand movement to manipulate that object. The current results add an additional layer of complexity to this picture by demonstrating that AIP, like PMv receives disynaptic input from cerebellum (as well as from the basal ganglia). The cerebellum is thought to be necessary for the adaptive adjustment of motor output and sensorimotor coordination. These mechanisms could have great utility for adjusting hand shape during object manipulation.
Summary of cerebellar and basal ganglia projections to AIP, PMv and M1. Heavy arrows indicate primary projections from subcortical sites. Dotted arrows indicate a less dense but significant projection. Note that AIP is unique in receiving projections from cerebellar output channels that are mainly directed at other cortical areas.
Nigral Input to AIP
Retrograde transneuronal transport of rabies virus from AIP injections demonstrated that AIP is a target of output from the basal ganglia. Specifically, we found that AIP receives a projection (via the thalamus) from neurons located in the caudal two-thirds of SNpr. This portion of SNpr also contains some neurons that project to regions of prefrontal cortex (Middleton and Strick, 2002). Because of the complex topography within SNpr, it is difficult to determine whether nigral neurons that project to AIP are segregated from or intermingled with those that project to regions of prefrontal cortex. On the other hand, it is clear that the basal ganglia neurons that project to AIP are entirely segregated from those that project to the cortical motor areas. The cortical motor areas receive basal ganglia input largely from the internal segment of the globus pallidus (GPi) (Hoover and Strick, 1993, 1999; Dum and Strick, 1999; Akkal et al., 2001, 2002). Only the face area of M1 receives some input from neurons located at mid-rostrocaudal levels of SNpr (Hoover and Strick, 1999). However, these SNpr neurons are rostral and dorsolateral to those that project to AIP.
AIP is densely interconnected with the PMv at the cortical level. An obvious question is whether they receive subcortical input from the same sources or even from the same output channels. The answer to this question differs for cerebellar and basal ganglia systems. As noted above, the broadly distributed field of dentate neurons that project to AIP overlaps the dentate output channel to the PMv. In contrast, AIP and PMv receive input from non-overlapping regions of the basal ganglia. PMv is the target of output from GPi, whereas AIP is the target of output from SNpr. These observations suggest that AIP is the target of a separate nigral output channel which is likely to convey information that is functionally distinct from that sent to the PMv and other cortical motor areas.
The demonstration of a nigral input (via the thalamus) to a region of posterior parietal cortex is perhaps the most surprising aspect of the current findings. Classically, the basal ganglia have long been known to receive input from multiple cortical areas including from posterior parietal cortex (e.g. Kemp and Powell, 1971). However, the output of the basal ganglia had been thought to influence primarily motor regions of the cerebral cortex. It is now generally accepted that the output of the basal ganglia also targets regions of prefrontal orbitofrontal and cingulate cortex (for references and review, see Alexander et al., 1986; Middleton and Strick, 2002). We have also provided evidence that the output of the basal ganglia projects to regions of inferotemporal cortex (Middleton and Strick, 1996). The current results reveal that the basal ganglia have an even broader sphere of influence than previously thought. Our report is the first demonstration of an anatomical pathway linking basal ganglia output with the parietal lobe in a primate.
The existence of a nigral projection to AIP clearly expands the potential sphere of influence of the basal ganglia. More importantly, this input may provide an explanation for some of the non-motor deficits observed in patients with Parkinson's disease (PD). Although PD is characterized by motor symptoms, many PD patients display deficits that involve aspects of visuomotor and visuospatial integration (Stern et al., 1983; Boller et al., 1984; Richards et al., 1993; Cronin-Golomb and Braun, 1997; Hocherman and Giladi, 1998; Lee et al., 2001a,b). For example, some PD patients show difficulty on tasks requiring them to mentally rotate a figure before copying it (de Jong et al., 1999). PD patients can also display deficits in making accurate assessments of vertical and horizontal, even when these judgements are reported verbally rather than manually (Danta and Hilton, 1975; Trick et al., 1994; Finton et al., 1998; Montse et al., 2001). These deficits are normally ascribed to dysfunction of posterior parietal cortex. The presence of a disynaptic projection from the nigra to a region of posterior parietal cortex provides an anatomical route to explain these otherwise paradoxical findings.
We thank M. Page for the development of computer programs and M. O'Malley and C. Lovell for their expert technical assistance. We also thank Drs C. Rupprecht (CDC, Atlanta, GA) for supplying the CVS-11 strain of rabies and A. Wandeler (Animal Diseases Research Institute, Nepean, Ontario, Canada) for supplying antibodies to rabies. This work was supported by the Veterans Affairs Medical Research Service and by US Public Health Service grants NS24328 (P.L.S.) and MH56661 (P.L.S.).
References
Akkal D, Dum RP, Strick PL (
Akkal D, Dum RP, Strick PL (
Alexander GE, DeLong MR, Strick PL (
Barrett AM, Crucian GP, Schwartz R, Nallamshetty H, Heilman KM (
Binkofski F, Buccino G, Stephan KM, Rizzolatti G, Seitz RJ, Freund HJ (
Boller F, Passafiume D, Keefe NC, Rogers K, Morrow L, Kim Y (
Chavis DA, Pandya DN (
Clower DM, West RA, Lynch JC, Strick PL (
Clower DM, Dum RP, Strick PL (
Clower DM, Dum RP, Strick PL (
Cronin-Golomb A, Braun AE (
Danta G, Hilton RC (
de Jong BM, Frackowiak RS, Willemsen AT, Paans AM (
Dum RP, Strick PL (
Dum RP, Strick PL (
Dum RP, Strick PL (
Dum RP, Li C, Strick PL (
Finton MJ, Lucas JA, Graff-Radford NR, Uitti RJ (
Francois C, Percheron G, Yelnik J (
Ghosh S, Gattera R (
Godschalk M, Lemon RN (
Godschalk M, Lemon RN, Kuypers HG, Ronday HK (
He SQ, Dum RP, Strick PL (
He SQ, Dum RP, Strick PL (
Hocherman S, Giladi N (
Holsapple JW, Preston JB, Strick PL (
Hoover JE, Strick PL (
Jancke L, Kleinschmidt A, Mirzazade S, Shah NJ, Freund HJ (
Kakei S, Shinoda Y (
Kakei S, Yagi J, Wannier T, Na J, Shinoda Y (
Kelly RM and Strick PL (
Kelly RM, Strick PL (
Kemp JM, Powell TP (
Langer LF, Graybiel AM (
Lee AC, Harris JP, Atkinson EA, Fowler MS (
Lee AC, Harris JP, Atkinson EA, Fowler MS (
Lewis JW, Van Essen DC (
Luppino G, Murata A, Govoni P, Matelli M (
Lynch JC, Hoover JE, Strick PL (
Matelli M, Camarda R, Glickstein M, Rizzolatti G (
Matelli M, Camarda R, Glickstein M, Rizzolatti G (
Mecklinger A, Gruenewald C, Besson M, Magnie MN, Von Cramon DY (
Middleton FA, Strick PL (
Middleton FA, Strick PL (
Middleton FA, Strick PL (
Middleton FA, Strick PL (
Middleton FA, Strick PL (
Montse A, Pere V, Carme J, Francesc V, Eduardo T (
Murata A, Gallese V, Kaseda M, Sakata H (
Murata A, Fadiga L, Fogassi L, Gallese V, Raos V, Rizzolatti G (
Murata A, Gallese V, Luppino G, Kaseda M, Sakata H (
Neal JW, Pearson RC, Powell TP (
Oztop E, Arbib MA (
Percheron G, Francois C, Talbi B, Yelnek J, Fenelon G (
Petrides M, Pandya DN (
Preuss TM, Goldman-Rakic PS (
Richards M, Cote LJ, Stern Y (
Rosene DL, Mesulam MM (
Rosene DL, Roy N, Davis BJ (
Sakata H, Taira M, Murata A, Mine S (
Sakata H, Taira M, Kusunoki M, Murata A, Tanaka Y (
Sakata H, Taira M, Kusunoki M, Murata A, Tanaka Y, Tsutsui K (
Sasaki K, Kawaguchi S, Oka H, Sakai M, Mizuno N (
Stern Y, Mayeux R, Rosen J, Ilson J (
Stoeckel MC, Weder B, Binkofski F, Buccino G, Shah NJ, Seitz RJ (
Strick PL, Card JP (
Strick PL, Preston JB (
Strick PL, Preston JB (
Suzuki H, Azuma M (
Taira M, Mine S, Georgopoulos AP, Murata A, Sakata H (
Tanne-Gariepy J, Rouiller EM, Boussaoud D (
Trick GL, Kaskie B, Steinman SB (
Wannier T, Kakei S, Shinoda Y (
Author notes
1Pittsburgh Veterans Affairs Medical Center, University of Pittsburgh School of Medicine, Pittsburgh, PA, USA, 2Center for the Neural Basis of Cognition, University of Pittsburgh School of Medicine, Pittsburgh, PA, USA and 3Departments of Neurobiology, Neurosurgery and Psychiatry, University of Pittsburgh School of Medicine, Pittsburgh, PA, USA