-
PDF
- Split View
-
Views
-
Cite
Cite
Giuseppe Stabile, Emanuele Bertaglia, Gaetano Senatore, Antonio De Simone, Franco Zoppo, Giovanni Donnici, Pietro Turco, Pietro Pascotto, Massimo Fazzari, Dino Franco Vitale, Catheter ablation treatment in patients with drug-refractory atrial fibrillation: a prospective, multi-centre, randomized, controlled study (Catheter Ablation For The Cure Of Atrial Fibrillation Study), European Heart Journal, Volume 27, Issue 2, January 2006, Pages 216–221, https://doi.org/10.1093/eurheartj/ehi583
Close - Share Icon Share
Abstract
Aims We conducted a multi-centre, prospective, controlled, randomized trial to investigate the adjunctive role of ablation therapy to antiarrhythmic drug therapy in preventing atrial fibrillation (AF) relapses in patients with paroxysmal or persistent AF in whom antiarrhythmic drug therapy had already failed.
Methods and results One hundred and thirty seven patients were randomized to ablation and antiarrhythmic drug therapy (ablation group) or antiarrhythmic drug therapy alone (control group). In the ablation group, patients underwent cavo-tricuspid and left inferior pulmonary vein (PV)-mitral isthmus ablation plus circumferential PV ablation. The primary end-point of the study was the absence of any recurrence of atrial arrhythmia lasting >30 s in the 1-year follow-up period, after 1-month blanking period. Three (4.4%) major complications were related to ablation: one patient had a stroke during left atrium ablation, another suffered transient phrenic paralysis, and the third had a pericardial effusion which required pericardiocentesis. After 12 months of follow-up, 63/69 (91.3%) control group patients had at least one AF recurrence, whereas 30/68 (44.1%) (P<0.001) ablation group patients had atrial arrhythmia recurrence (four patients had atrial flutter, 26 patients AF).
Conclusion Ablation therapy combined with antiarrhythmic drug therapy is superior to antiarrhythmic drug therapy alone in preventing atrial arrhythmia recurrences in patients with paroxysmal or persistent AF in whom antiarrhythmic drug therapy has already failed.
See page 130 for the editorial comment on this article (doi:10.1093/eurheartj/ehi625)
Introduction
Catheter ablation has been proposed as a promising technique to treat atrial fibrillation (AF), either as an alternative or combined to antiarrhythmic therapy, as the latter is usually ineffective in preventing both arrhythmia recurrences and clinical events related to them.1,2 However, most trials evaluating the efficacy of the ablation strategy are single-centre, uncontrolled, observational studies. Moreover, the criteria used to define the success are not well defined and often depend on patients' symptoms. To overcome these limitations, we conducted a multi-centre, prospective, controlled, randomized trial to investigate the adjunctive role of ablation therapy to antiarrhythmic drug therapy in preventing AF relapses in patients with paroxysmal or persistent AF in whom antiarrhythmic drug therapy had already failed.
Methods
Patient population
Between February 2002 and June 2003, 137 out of 207 consecutive patients referred to our centres for AF catheter ablation were enrolled. We included patients with paroxysmal or persistent AF who were intolerant of antiarrhythmic drugs or in whom two or more antiarrhythmic drug regimens had failed. Paroxysmal AF was defined as the occurrence, in the previous 6 months, of one or more episodes of AF a month, each lasting more than 60 min but less than 7 days,3 with all episodes terminating spontaneously. Persistent AF was defined as the occurrence, in the previous 12 months, of two or more episodes of AF, each lasting more than 7 days before being terminated pharmacologically or by electrical cardioversion, or lasting less than 7 days but necessitating early cardioversion owing to intolerable symptoms or haemodynamic compromise, with sinus rhythm maintained for 60 min or more after termination. In all patients, the first diagnosis of AF had been made at least 6 months before enrolment.
Exclusion criteria were: (1) age <18 or >80 years; (2) permanent AF (AF was the sole rhythm for the last 12 months); (3) AF secondary to a transient or correctable abnormality, including electrolyte imbalance, trauma, recent surgery, infection, toxic ingestion, and endocrinopathy; (4) persistence of AF episodes triggered by another uniform arrhythmia (i.e. atrial flutter or atrial tachycardia) despite previous supraventricular tachycardia ablation; (5) intra-atrial thrombus, tumour, or other abnormality precluding catheter insertion; (6) Wolff–Parkinson–White syndrome; (7) heart failure with NYHA class III or IV or EF ≤35%; (7) unstable angina or acute myocardial infarction within 3 months; (8) cardiac revascularization or other cardiac surgery within 6 months or with prior atrial surgery; (9) renal failure requiring dialysis, or hepatic failure; (10) an implanted device (pacemaker or cardioverter-defibrillator); (11) left atrial diameter >60 mm.
Study protocol
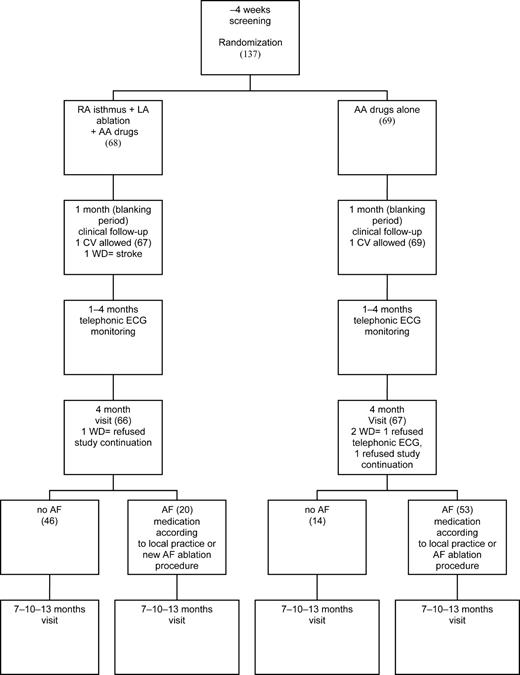
The study was approved by our institutional review committee, and the patients gave written informed consent to be enrolled. Figure 1 summarizes the flowchart of the study. Enrolled patients were randomized to ablation plus antiarrhythmic drug therapy (ablation group) or antiarrhythmic drug therapy alone (control group). Randomization was computer generated at the statistical analysis coordinating centre (Fondazione S. Maugeri, Telese, BN, Italy). The antiarrhythmic drug preferentially administered was amiodarone. In patients with a history of side-effects or intolerance to amiodarone, a class IC antiarrhythmic drug was administered. The final decision was left to the physician in accordance with local practice. Most patients received oral anticoagulation therapy with warfarin sodium to achieve an international normalized ratio of 2–3. Patients who were intolerant or who refused anticoagulation therapy received antiaggregant therapy (acetylsalicylic acid or ticlopidine). After a 1-month blanking period, patients received a transtelephonic ECG recorder (Sorin Life Watch, Italy), and a 30 s ECG was scheduled each day for 3 months. Moreover, patients were instructed to obtain an ECG in the event of palpitations. Standard ECG, Holter monitoring, and transthoracic echocardiography were scheduled at 1, 4, 7, 10, and 13 months. In ablation group patients, transoesophageal echocardiography was scheduled 4 months after ablation, to evaluate pulmonary vein (PV) Doppler flow velocity. PV stenosis was defined by a peak velocity of ≥110 cm/s and the presence of turbulence and deformity of flow signal.4,5 Because recurrences of atrial tachyarrhythmias within the first month after ablation may be a transient phenomenon, this time interval (1-month blanking period) was excluded from the analysis. Transtelephonic ECG and Holter monitoring were analysed and interpreted by two independent physicians unaware of the randomly assigned treatment; in the event of disagreement, the final interpretation was left to one of the authors, who was unaware of which group the patient belonged to.
Catheter ablation
Left atrium and PVs were explored using a trans-septal approach. Real-time 3D left atrium maps were reconstructed using a non-fluoroscopic navigation system (CARTO, Biosense-Webster Inc., Diamond Bar, California). Maps were acquired during pacing from the coronary sinus. In patients with AF at the beginning of the procedure, cardioversion was performed before starting 3D mapping. Radiofrequency pulses were delivered using an 8 mm tip catheter (with a temperature setting of 60°C and a radiofrequency energy up to 100 W) in the first 17 patients, and a 3.5 mm cooled-tip catheter (with a temperature setting up to 45°C and a radiofrequency energy up to 50 W) in the remaining patients. When ablation was performed in the posterior wall, radiofrequency power was reduced to 50 or 25 W, using the 8 and 3.5 mm tip catheter, respectively, to reduce the risk of injuring the surrounding structure. In both cases, radiofrequency energy was delivered for up to 120 s until local electrogram amplitude was reduced ≥80%. To reduce the risk of the catheter tip overheating, a fine back-and-forth movement of the catheter was adopted during radiofrequency delivery at the ablation site. The ablation lines consisted of contiguous focal lesions deployed at a distance ≥5 mm from the ostia of the PVs, creating a circumferential line around each PV. Another ablation line was created by connecting the left inferior PV to the mitral annulus (mitral isthmus). Remapping was performed in all patients in sinus rhythm, during coronary sinus pacing, using the pre-ablation anatomic map for acquisition of new points. The end-point of the ablation procedure was low peak-to-peak bipolar potentials (<0.1 mV) inside the lesion, as determined by local electrogram analysis and voltage maps. A minimum of five points for each circumferential line was sampled. If sites of high voltage (>0.1 mV) were still present, additional ablations were performed, both along the encircling ablation lines and within them. The block along the line connecting the mitral valve to lower lateral PV was evaluated by pacing from the distal electrodes of the coronary sinus catheter; the end-point of the ablation was the recording on the distal electrodes of the ablation catheter, positioned on that line, of a double potential with an isoelectric line of at least 80 ms.
Patients with conduction along the cavo-tricuspid isthmus underwent inferior vena cava-tricuspid annulus isthmus ablation at the end of left atrium ablation, in a single session.
All patients received effective oral anticoagulation (international normalized ration between 2 and 3) for ≥1 month before ablation. Heparin anticoagulation replaced oral anticoagulants ≥72 h before ablation, and was stopped 4 h before the procedure. After trans-septal puncture, an intravenous bolus of heparin (5000 IU) was administered, followed by infusion or additional boluses to maintain an activated clotting time ≥250 s. Oral anticoagulation was usually restarted before hospital discharge.
Statistical analysis
Most antiarrhythmic drugs are able to maintain sinus rhythm in only ∼50% of patients at 1 year.1 For amiodarone, this percentage appears to be significantly greater.6,7 In AF catheter ablation studies using a pure anatomical approach to encircle the PVs, a success rate of over 80% has been reported in patients taking previously ineffective drugs.8–10 Therefore, to calculate the number of patients to be enrolled, we defined as meaningful a 30% difference in atrial arrhythmia recurrence between the two groups, while alpha and a beta were set at 0.05. To the estimated sample size of 132 patients (66 in each branch), we added five more patients to compensate for possible drop outs; the final sample, therefore, comprised 137 patients. Data are reported as mean±standard deviation and median (25th, 75th percentiles). Student t-test for unpaired data and Fisher exact test were used, as appropriate. Two-tailed tests of significance are reported, and P-values <0.05 are considered statistically significant. The Benjamini and Hochberg False Discovery Rate correction was adopted to adjust P-values for multiple testing bias. Kaplan–Meier event-free survival curves and the Cox proportional hazard regression model were used to analyse factors influencing the time to first occurrence of atrial arrhythmia. Assigned treatment and all the clinical factors marked by an asterisk and reported in Table 1 were tested in the model using both LR forward and backward selection procedures. A dichotomous variable was considered for inclusion in the model if its occurrence in the whole population was >4%. The final model was the same regardless of the selection procedure used. Proportional hazard assumption was assessed by visual judgement of the log-minus-log survival plots and by checking the significance of a time-dependent variable added to the model (time-dependent variable=product of the variable under inquiry for proportional hazard assumption and the time). Linearity assumption for continuous variables was assessed by checking the significance of the transformed variable added to the model. Transforming was achieved by taking the natural logarithm and the square of the variable. Both linearity and proportional hazard assumptions were met in the final model. The primary end-point of the study was the absence of any recurrence of atrial arrhythmia lasting >30 s in the 1-year follow-up period, after the 1-month blanking period. The primary outcome of the study was evaluated according to the intention-to-treat principle.
Results
Patient characteristics
Table 1 summarizes the clinical characteristics of the two study groups and drug distribution after randomization. The mean doses of antiarrhythmic drugs were: 209±49 mg amiodarone, 191±28 mg flecainide, 750±126 mg propafenone, 184±54 mg sotalol, and 500 mg disopyramide in the only patient who took this drug. Twenty three (34%) ablation group patients, and 21 (30%) control group patients were treated with a drug that had previously failed. In both groups, antiarrhythmic therapy was changed after randomization only if patients experienced an atrial arrhythmia recurrence; this did not, therefore, affect the study end-point.
Catheter ablation
All ablation group patients underwent a single AF ablation session. Mean left atrium ablation procedure time was 193±66 (range 91–350) min, with a mean fluoroscopic time of 25±10 (11–45) min, and a mean delivered energy of 121441±36865 (73800–189000) J. Cavo-tricuspid isthmus ablation was successful in 55/55 ablation group patients; the remaining 13 patients had no conduction along the isthmus, as they had already undergone cavo-tricuspid isthmus ablation. The end-point of mitral isthmus ablation was reached in only 21/68 (30.8%) patients; the mean interval between the two components of separated double potentials, during coronary sinus pacing, was 139±29 (95–203) ms. Three (4.4%) major complications were related to ablation: one patient had a stroke during left atrium ablation, another suffered transient phrenic paralysis, and a third had a pericardial effusion which required pericardiocentesis.
Clinical outcomes
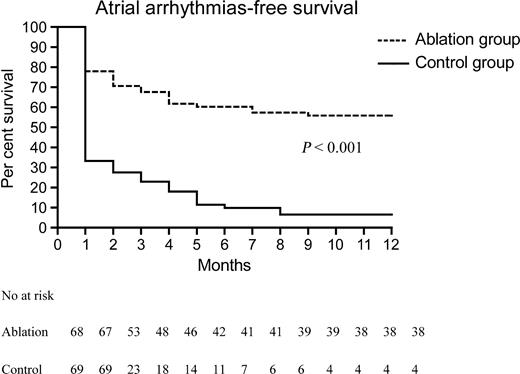
In the blanking period, 24/68 (35.3%) ablation group patients had an atrial arrhythmia recurrence, compared with 49/69 (71%) (P<0.001) control group patients; among these, six (25%) ablation group patients and 11 (22.4%) (P=0.81) control group patients required electrical cardioversion. In the 12 months after the blanking period, 63/69 (91.3%) control group patients had at least one AF recurrence, whereas 30/68 (44.1%) (P<0.001) ablation group patients had atrial arrhythmia recurrences (four patients had atrial flutter, 26 patients AF). No significant difference (P=0.64) was observed in atrial arrhythmia recurrence rates between patients in whom ablation was performed by means of an 8 mm tip catheter (8/17 patients, 47.1%) and those in whom a cooled-tip catheter was used (22/51 patients, 43.1%). In the ablation group, five out of 30 (16.7%) patients, who had experienced at least one atrial arrhythmia recurrence, had a single episode of atrial arrhythmia (four AF, one atrial flutter) during the first 3 months, and no further arrhythmia was documented during the follow-up. In the 3 months in which patients were monitored by means of transtelephonic ECG recorder, 30 patients had at least one asymptomatic AF recurrence. The final Cox proportional hazard model evaluating predictors of AF recurrence shows as significant only the ablation/medical therapy factor (hazard ratio for medical therapy equal to 3.2, 95% confidence interval=2.0–5.1). None of the clinical factors listed in Table 1 had a significant impact on the Cox model, even when the model was restricted to the subgroup of patients randomized to ablation therapy. Figure 2 shows Kaplan–Meier plots relative to the ablation and control groups. Among 63 control group patients with AF relapses, 36 (57.1%) underwent catheter ablation, while continuing the previous ineffective antiarrhythmic drug regime, 3 (2–4) months after AF recurrence. After a median follow-up of 18 (14–23) months, 22 (61.1%) patients did not experience further atrial arrhythmia recurrence. During the follow-up, one ablation group patient underwent percutaneous coronary angioplasty 3 months after ablation. No PV stenosis was reported. Two ablation group patients were intolerant to amiodarone and flecainide, respectively, 6 and 4 months after randomization, and were put on flecainide and sotalol, respectively. In the control group, one patient had a transient ischaemic attack, two patients had cancer (one died), and one patient suffered sudden death; all these patients had already experienced an AF recurrence. In the ablation group, the patient who had stroke during ablation died of a brain haemorrhage 9 months later. During the 1-year follow-up there was no statistically significant difference in the median per-patient number of hospitalizations, including that required for ablation, between the ablation, 1 (1–2), and control, 2 (1–2), group (P=0.34).
Discussion
This is the first prospective, randomized, multi-centre, controlled study to demonstrate that ablation therapy combined with antiarrhythmic drugs is more efficacious than antiarrhythmic drug therapy alone in patients with paroxysmal or persistent AF in whom antiarrhythmic drug therapy has already failed. This result was obtained by means of a single AF ablation procedure. Moreover, for the first time, the efficacy of ablation therapy was assessed by means of over 90 ECG per patient, obtained through daily transtelephonic ECG monitoring.
All but one11 study on AF catheter ablation have used surrogate end-points,12 such as time to first symptomatic recurrence, to define the success of the ablation procedure. We also chose the time to first atrial arrhythmia recurrence as the primary end-point. However, we made a great effort to detect the largest possible number of AF recurrences, both symptomatic and asymptomatic, by means of multiple ECG and Holter monitoring, and, especially, by daily transtelephonic recording of ECGs. This strategy might explain the lower success rate (56 vs. 80–90%) reported in our study than in others that used a purely anatomical approach;10,11,13 indeed, the use of transtelephonic monitoring enables better recognition of brief or asymptomatic AF episodes, and this might affect the clinical outcome of patients with AF.14 In patients who underwent AF catheter ablation by means of a purely anatomical approach, transtelephonic monitoring showed a high number of asymptomatic AF recurrences (in the series of Senatore et al.15 50% of patients with recurrences were asymptomatic) in the short-term follow-up, whereas recurrences were infrequent in the long-term follow-up.16,17
Other parameters which may influence the success rate of AF ablation are the type of AF (paroxysmal or persistent) and the clinical characteristics of the study population. Early experiences of AF ablation involved patients with paroxysmal AF and a low incidence of structural heart disease,18,19 whereas later studies8,20 also included patients with persistent AF (up to 29% of the study population) and a higher incidence of heart disease (up to 27%). In our study population, one-third of patients had persistent AF and most had heart disease. Finally, only by using a control group we can assess the true impact of the ablation strategy in the management of AF. In the only study11 in which, in a non-randomized trial, ablation was compared with medical therapy, the percentages of AF-free patients were 84, 79, and 78%, respectively, at 1, 2, and 3 years among ablated patients, and 61, 47, and 37%, respectively, among those medically treated. The very low proportion of AF-free patients in the medical therapy group observed in our study is likely related to our more intensive monitoring strategy and may also be related to enrolment of a more severely ill population. Wazni et al.21 recently reported the results of a randomized trial in which AF ablation was tested as first-line therapy for treating patients with symptomatic AF.21 In their series, at the end of a 1-year follow-up, 87% of ablated patients were free of symptomatic AF recurrence, compared with 37% of patients who received antiarrhythmic drugs. The absolute per cent improvement achieved by ablation therapy vs. antiarrhythmic therapy was 50 points. This is similar to our data (47 points) and suggests that, whatever ablation strategy is used, the success rate of ablation mainly depends on the clinical characteristics of the patients treated. The main limitation of AF ablation is the high incidence of major complications, in comparison with those reported during the ablation of other supraventricular tachycardia. A recent survey22 of over 6000 European patients who underwent AF ablation reported an average 51±22% cure of AF, without drugs, whereas PV stenosis was recorded in 1.85±3% of the patients, strokes in 1±2%, and tamponade in 1.9±2.55%. In a worldwide survey23 of 8745 patients in whom antiarrhythmic drugs had proved ineffective, 75.9% became asymptomatic after AF ablation, while major complications were reported in 6%. In our population, we observed a 4.4% incidence of major complications related to the ablation procedure.
Limitations
This study has several potential limitations: (1) The interval chosen to define the conduction block across the mitral isthmus was arbitrary, and the completeness of conduction block across the ablation line was not routinely assessed. However, the incidence of left atrial flutter was very low, and similar data were reported by Oral et al.10 and by Kottkamp et al.,24 neither of whom routinely assessed the completeness of conduction block. (2) Daily transtelephonic ECG monitoring was used only for 3 months after the blanking period; although most AF recurrences occur in this period,17,24,25 some brief or asymptomatic long-term recurrences might have gone undiagnosed. (3) Although over 60% of our patients had a structural heart disease, most of them had well-preserved systolic function. Our data cannot, therefore, be extrapolated to patients with more severe heart disease and impaired systolic function. (4) Owing to the limited sample size and follow-up period, we were not able to evaluate the impact of the two therapeutic strategies on clinical end-points such as death, stroke, hospitalization, and so on.
Clinical implications
In the latest ACC/AHA/ESC guidelines26 for the management of patients with AF, catheter ablation was not considered among the therapeutic strategies in patients with drug-refractory AF. If further prospective, randomized, controlled studies on larger population should confirm the superiority of the ablation therapy, combined with antiarrhythmic drugs or not, over antiarrhythmic drugs alone, catheter ablation could well be recognized as a step in the management of AF.
Acknowledgements
The authors thank Drs Marcella Jorfida and Luca Oselladore for their work in analysing transtelephonic ECG, and Alessandro Dulio, BS, and Gabriele Fischetto, BS, from Biosense-Webster, Italy, for their technical support. The trial was partly supported by Biosense-Webster, Italy.
Conflict of interest: none declared.
The trial was presented as a late-breaking trial at the 2005 Scientific Session of the American College of Cardiology in Orlando.
Figure 1 Study protocol. RA, right atrium; LA, left atrium; CV, cardioversion; ECG, electrocardiogram; AA,antiarrhythmic; WD, withdrawal. In brackets patients number in each stage.
Figure 2 Atrial arrhythmia-free survival curves after the blanking period.
Clinical characteristics of study population
| . | Ablation group (n=68) . | Control group (n=69) . | P-value . | Benjamini and Hochberg adjusted P-value . |
|---|---|---|---|---|
| Age (years) | 62.2±9 | 62.3±10.7 | 0.97a | |
| Sex (male/female) | 37/31 | 44/25 | 0.27a | |
| Paroxysmal/persistent AF | 42/26 | 50/19 | 0.19a | |
| AF history (years) | 5.1±3.9 | 7.1±5.9 | 0.02a | 0.15 |
| LA diameter (mm) | 46±5 | 45.4±5.5 | 0.51a | |
| LVEF (%) | 59.1±6.7 | 57.9±5.8 | 0.25a | |
| Heart disease (%) | 43 (63.2) | 43 (62.3) | 0.82a | |
| Hypertension (%) | 36 (52.9) | 34 (49.3) | ||
| Dilated cardiomyopathy (%) | 1 (1.5) | 2 (2.9) | ||
| Ischaemic (%) | 3 (4.4) | 5 (7.2) | ||
| Valvular (%) | 3 (4.4) | 2 (2.9) | ||
| Previous SVT ablation (%) | 3 (4.4) | 3 (4.3) | 0.99a | |
| Previous CTI ablation (%) | 13 (14.5) | 21 (30.4) | 0.13a | |
| Previous RA linear ablation (%) | 4 (5.9) | 5 (7.2) | 0.75a | |
| Previous LA linear ablation (%) | 1 (1.5) | 1 (1.5) | 0.99a | |
| Amiodarone (%) | 45 (66.2) | 43 (62.3) | 0.64a | |
| Flecainide (%) | 17 (25) | 18 (26) | 0.88a | |
| Propafenone (%) | 5 (7.4) | 7 (10.1) | 0.57a | |
| Disopyramide (%) | 0 | 1 (1.5) | 0.32 | |
| Sotalol (%) | 1 (1.5) | 4 (5.8) | 0.18 | |
| ACE-inhibitors or angiotensin receptor blockers (%) | 29 (42.6) | 36 (52.1) | 0.27a | |
| Verapamil or diltiazem (%) | 5 (7.4) | 2 (2.9) | 0.24a | |
| β-Blockers (%) | 6 (8.8) | 7 (10.1) | 0.79a | |
| Statins (%) | 11 (16.2) | 10 (14.5) | 0.79a | |
| Oral anticoagulant (%) | 65 (95.6) | 56 (81.2) | 0.003a | 0.09 |
| Oral antiaggregant (%) | 3 (4.4) | 13 (18.8) | 0.008a | 0.07 |
| . | Ablation group (n=68) . | Control group (n=69) . | P-value . | Benjamini and Hochberg adjusted P-value . |
|---|---|---|---|---|
| Age (years) | 62.2±9 | 62.3±10.7 | 0.97a | |
| Sex (male/female) | 37/31 | 44/25 | 0.27a | |
| Paroxysmal/persistent AF | 42/26 | 50/19 | 0.19a | |
| AF history (years) | 5.1±3.9 | 7.1±5.9 | 0.02a | 0.15 |
| LA diameter (mm) | 46±5 | 45.4±5.5 | 0.51a | |
| LVEF (%) | 59.1±6.7 | 57.9±5.8 | 0.25a | |
| Heart disease (%) | 43 (63.2) | 43 (62.3) | 0.82a | |
| Hypertension (%) | 36 (52.9) | 34 (49.3) | ||
| Dilated cardiomyopathy (%) | 1 (1.5) | 2 (2.9) | ||
| Ischaemic (%) | 3 (4.4) | 5 (7.2) | ||
| Valvular (%) | 3 (4.4) | 2 (2.9) | ||
| Previous SVT ablation (%) | 3 (4.4) | 3 (4.3) | 0.99a | |
| Previous CTI ablation (%) | 13 (14.5) | 21 (30.4) | 0.13a | |
| Previous RA linear ablation (%) | 4 (5.9) | 5 (7.2) | 0.75a | |
| Previous LA linear ablation (%) | 1 (1.5) | 1 (1.5) | 0.99a | |
| Amiodarone (%) | 45 (66.2) | 43 (62.3) | 0.64a | |
| Flecainide (%) | 17 (25) | 18 (26) | 0.88a | |
| Propafenone (%) | 5 (7.4) | 7 (10.1) | 0.57a | |
| Disopyramide (%) | 0 | 1 (1.5) | 0.32 | |
| Sotalol (%) | 1 (1.5) | 4 (5.8) | 0.18 | |
| ACE-inhibitors or angiotensin receptor blockers (%) | 29 (42.6) | 36 (52.1) | 0.27a | |
| Verapamil or diltiazem (%) | 5 (7.4) | 2 (2.9) | 0.24a | |
| β-Blockers (%) | 6 (8.8) | 7 (10.1) | 0.79a | |
| Statins (%) | 11 (16.2) | 10 (14.5) | 0.79a | |
| Oral anticoagulant (%) | 65 (95.6) | 56 (81.2) | 0.003a | 0.09 |
| Oral antiaggregant (%) | 3 (4.4) | 13 (18.8) | 0.008a | 0.07 |
Values are presented as mean±SD or number of patients.
aVariables included in the Cox proportional hazards model.
LVEF, left ventricular ejection fraction; SVT, supraventricular tachycardia; CTI, cavo-tricuspid isthmus; ACE, angiotensin-converting enzyme.
Clinical characteristics of study population
| . | Ablation group (n=68) . | Control group (n=69) . | P-value . | Benjamini and Hochberg adjusted P-value . |
|---|---|---|---|---|
| Age (years) | 62.2±9 | 62.3±10.7 | 0.97a | |
| Sex (male/female) | 37/31 | 44/25 | 0.27a | |
| Paroxysmal/persistent AF | 42/26 | 50/19 | 0.19a | |
| AF history (years) | 5.1±3.9 | 7.1±5.9 | 0.02a | 0.15 |
| LA diameter (mm) | 46±5 | 45.4±5.5 | 0.51a | |
| LVEF (%) | 59.1±6.7 | 57.9±5.8 | 0.25a | |
| Heart disease (%) | 43 (63.2) | 43 (62.3) | 0.82a | |
| Hypertension (%) | 36 (52.9) | 34 (49.3) | ||
| Dilated cardiomyopathy (%) | 1 (1.5) | 2 (2.9) | ||
| Ischaemic (%) | 3 (4.4) | 5 (7.2) | ||
| Valvular (%) | 3 (4.4) | 2 (2.9) | ||
| Previous SVT ablation (%) | 3 (4.4) | 3 (4.3) | 0.99a | |
| Previous CTI ablation (%) | 13 (14.5) | 21 (30.4) | 0.13a | |
| Previous RA linear ablation (%) | 4 (5.9) | 5 (7.2) | 0.75a | |
| Previous LA linear ablation (%) | 1 (1.5) | 1 (1.5) | 0.99a | |
| Amiodarone (%) | 45 (66.2) | 43 (62.3) | 0.64a | |
| Flecainide (%) | 17 (25) | 18 (26) | 0.88a | |
| Propafenone (%) | 5 (7.4) | 7 (10.1) | 0.57a | |
| Disopyramide (%) | 0 | 1 (1.5) | 0.32 | |
| Sotalol (%) | 1 (1.5) | 4 (5.8) | 0.18 | |
| ACE-inhibitors or angiotensin receptor blockers (%) | 29 (42.6) | 36 (52.1) | 0.27a | |
| Verapamil or diltiazem (%) | 5 (7.4) | 2 (2.9) | 0.24a | |
| β-Blockers (%) | 6 (8.8) | 7 (10.1) | 0.79a | |
| Statins (%) | 11 (16.2) | 10 (14.5) | 0.79a | |
| Oral anticoagulant (%) | 65 (95.6) | 56 (81.2) | 0.003a | 0.09 |
| Oral antiaggregant (%) | 3 (4.4) | 13 (18.8) | 0.008a | 0.07 |
| . | Ablation group (n=68) . | Control group (n=69) . | P-value . | Benjamini and Hochberg adjusted P-value . |
|---|---|---|---|---|
| Age (years) | 62.2±9 | 62.3±10.7 | 0.97a | |
| Sex (male/female) | 37/31 | 44/25 | 0.27a | |
| Paroxysmal/persistent AF | 42/26 | 50/19 | 0.19a | |
| AF history (years) | 5.1±3.9 | 7.1±5.9 | 0.02a | 0.15 |
| LA diameter (mm) | 46±5 | 45.4±5.5 | 0.51a | |
| LVEF (%) | 59.1±6.7 | 57.9±5.8 | 0.25a | |
| Heart disease (%) | 43 (63.2) | 43 (62.3) | 0.82a | |
| Hypertension (%) | 36 (52.9) | 34 (49.3) | ||
| Dilated cardiomyopathy (%) | 1 (1.5) | 2 (2.9) | ||
| Ischaemic (%) | 3 (4.4) | 5 (7.2) | ||
| Valvular (%) | 3 (4.4) | 2 (2.9) | ||
| Previous SVT ablation (%) | 3 (4.4) | 3 (4.3) | 0.99a | |
| Previous CTI ablation (%) | 13 (14.5) | 21 (30.4) | 0.13a | |
| Previous RA linear ablation (%) | 4 (5.9) | 5 (7.2) | 0.75a | |
| Previous LA linear ablation (%) | 1 (1.5) | 1 (1.5) | 0.99a | |
| Amiodarone (%) | 45 (66.2) | 43 (62.3) | 0.64a | |
| Flecainide (%) | 17 (25) | 18 (26) | 0.88a | |
| Propafenone (%) | 5 (7.4) | 7 (10.1) | 0.57a | |
| Disopyramide (%) | 0 | 1 (1.5) | 0.32 | |
| Sotalol (%) | 1 (1.5) | 4 (5.8) | 0.18 | |
| ACE-inhibitors or angiotensin receptor blockers (%) | 29 (42.6) | 36 (52.1) | 0.27a | |
| Verapamil or diltiazem (%) | 5 (7.4) | 2 (2.9) | 0.24a | |
| β-Blockers (%) | 6 (8.8) | 7 (10.1) | 0.79a | |
| Statins (%) | 11 (16.2) | 10 (14.5) | 0.79a | |
| Oral anticoagulant (%) | 65 (95.6) | 56 (81.2) | 0.003a | 0.09 |
| Oral antiaggregant (%) | 3 (4.4) | 13 (18.8) | 0.008a | 0.07 |
Values are presented as mean±SD or number of patients.
aVariables included in the Cox proportional hazards model.
LVEF, left ventricular ejection fraction; SVT, supraventricular tachycardia; CTI, cavo-tricuspid isthmus; ACE, angiotensin-converting enzyme.
References
Wyse DG, Waldo AL, DiMarco JP, Domanski MJ, Rosenberg Y, Schron EB, Kellen JC, Greene HL, Mickel MC, Dalquist JE, Corley SD. A comparison of rate control and rhythm control in patients with atrial fibrillation.
Van Gelder IC, Hagens VE, Bosker HA, Kingma JH, Kamp O, Kingma T, Said SA, Darmanata JI, Timmermans AJ, Tijssen JG, Crijns HJ; Rate Control versus Electrical Cardioversion for Persistent Atrial Fibrillation Study Group. A comparison of rate control and rhythm control in patients with recurrent persistent atrial fibrillation.
Levy S, Camm AJ, Saksena S, Aliot E, Breithardt G, Crijns H, Davies W, Kay N, Prystowsky E, Sutton R, Waldo A, Wyse DG; Working Group on Arrhythmias, Working Group on Cardiac Pacing of the European Society of Cardiology, North American Society of Pacing and Electrophysiology. International consensus on nomenclature and classification of atrial fibrillation; a collaborative project of the Working Group on Arrhythmias and the Working Group on Cardiac Pacing of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology.
Yu WC, Hsu TL, Tai CT, Tsai CF, Hsieh MH, Lin WS, Lin YK, Tsao HM, Ding YA, Chang MS, Chen SA. Acquired pulmonary vein stenosis after radiofrequency catheter ablation of paroxysmal atrial fibrillation.
Arentz T, Jander N, von Rosenthal J, Blum T, Furmaier R, Gornadt L, Neuman FJ, Kalusche D. Incidence of pulmonary vein stenosis 2 years after radiofrequency catheter ablation of refractory atrial fibrillation.
Roy D, Talajic M, Dorian P, Connolly S, Eisenberg MJ, Green M, Kus T, Lambert J, Dubuc M, Gagne P, Nattel S, Thibault B. Amiodarone to prevent recurrences of atrial fibrillation.
Hohnloser SH, Kuck KH, Lilienthal J for the PIAF Investigators. Rhythm or rate control in atrial fibrillation—Pharmacological Intervention in Atrial Fibrillation (PIAF): a randomized trial.
Pappone C, Oreto G, Rosanio S, Vicedomini G, Tocchi M, Gugliotta F, Salvati A, Dicandia C, Calabro MP, Mazzone P, Ficarra E, Di Gioia C, Gulletta S, Nardi S, Santinelli V, Benussi S, Alfieri O. Atrial electroanatomical remodeling after circumferential radiofrequency pulmonary vein ablation. Efficacy of an anatomic approach in a large cohort of patients with atrial fibrillation.
Stabile G, Bertaglia E, Senatore G, De Simone A, Zerbo F, Carreras G, Turco P, Pascotto P, Fazzari M. Feasibility of pulmonary vein ostia radiofrequency ablation in patients with atrial fibrillation: a multicenter study (CACAF Pilot Study).
Oral H, Scharf C, Chugh A, Hall B, Cheung P, Good E, Veerareddy S, Pelosi F Jr, Morady F. Catheter ablation for paroxysmal atrial fibrillation. Segmental pulmonary vein ostial ablation versus left atrial ablation.
Pappone C, Rosanio S, Augello G, Gallus G, Vicedomini G, Mazzone P, Gulletta S, Gugliotta F, Pappone A, Santinelli V, Tortoriello V, Sala S, Zangrillo A, Crescenzi G, Benussi S, Alfieri O. Mortality, morbidity, and quality of life after circumferential pulmonary vein ablation for atrial fibrillation.
Wyse DG. Selection of endpoints in atrial fibrillation study.
Stabile G, Turco P, La Rocca V, Nocerino P, Stabile E, De Simone A. Is pulmonary vein isolation necessary for curing atrial fibrillation?
Glotzer TV, Hellkamp AS, Zimmerman J, Sweeney MO, Yee R, Marinchak R, Cook J, Paraschos A, Love J, Radoslovich G, Lee KL, Lamas GA. MOST Investigators. Atrial high rate episodes detected by pace-maker diagnostics predict death and stroke.
Senatore G, Stabile G, Bertaglia E, Donnici G, De Simone A, Zoppo F, Turco P, Pascotto P, Fazzari M. Role of transtelephonic electrocardiographic monitoring in detecting short-term arrhythmia recurrences after radiofrequency ablation in patients with atrial fibrillation.
Pappone C, Santinelli V, Manguso P, Vicedomini G, Gugliotta F, Augello G, Mazzone P, Tortoriello V, Landoni G, Zangrillo A, Lang C, Tomita T, Mesas C, Mastella E, Alfieri O. Pulmonary vein denervation enhances long-term benefit after circumferential ablation for paroxysmal atrial fibrillation.
Oral H, Veerareddy S, Good E, Hall B, Cheung P, Tamirisa K, Han J, Fortino J, Chugh A, Bogun F, Pelosi F Jr, Morady F. Prevalence of asymptomatic recurrences of atrial fibrillation after successful radiofrequency catheter ablation.
Haïssaguerre M, Jaïs P, Shah DC, Takahashi A, Hocini M, Quiniou G, Garrigue S, Le Mouroux A, Le Metayer P, Clementy J. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins.
Chen SA, Hsieh MH, Tai CT, Tsai CF, Prakash VS, Yu WC, Hsu TL, Ding YA, Chang MS. Initiation of atrial fibrillation by ectopic beats originating from the pulmonary veins. Electrophysiological characteristics, pharmacological responses, and effects of radiofrequency ablation.
Saad EB, Rossillo A, Saad CP, Martin DO, Bhargava M, Erciyes D, Bash D, Williams-Andrews M, Beheiry S, Marrouche NF, Adams J, Pisano E, Fanelli R, Potenza D, Raviele A, Bonso A, Themistoclakis S, Brachmann J, Saliba WI, Schweikert RA, Natale A. Pulmonary vein stenosis after radiofrequency ablation of atrial fibrillation.
Wazni OM, Marrouche NF, Martin DO, Verma A, Bhargava M, Saliba W, Bash D, Schweikert R, Brachmann J, Gunther J, Gutleben K, Pisano E, Potenza D, Fanelli F, Raviele A, Themistoclakis S, Rossillo A, Bonso A, Natale A. Radiofrequency ablation vs antiarrhythmic drugs as first-line treatment of symptomatic atrial fibrillation.
Jais P, Pappone C, Kuck KH, Tondo C, Schmitt C, Arentz T, Cauchemez B, Scaglione M, Montenero AS, Pisapia A, Furniss S, Haissaguerre M. European experience of atrial fibrillation ablation. (Abstract).
Cappato R, Calkins H, Chen SA, Davies W, Iesaka Y, Kalman J, Kim YO, Klein G, Packer D, Skanes A. Worldwide Survey on the Methods, Efficacy, and Safety of Catheter Ablation for Human Atrial Fibrillation.
Kottkamp H, Tanner H, Kobza R, Schirdewahn P, Dorszewski A, Gerds-Li JH, Carbucicchio C, Piorkowski C, Hindricks G. Time course of quantitative analysis of atrial fibrillation episode number and duration after circular plus linear left atrial lesions.
Hsieh MH, Tai CT, Tsai CF, Lin WS, Lin YK, Tsao HM, Huang JL, Ueng KC, Yu WC, Chan P, Ding YA, Chang MS, Chen SA. Clinical outcome of very late recurrence of atrial fibrillation after catheter ablation of paroxysmal atrial fibrillation.
Fuster V, Ryden LE, Asinger RW, Cannom DS, Crijns HJ, Frye RL, Halperin JL, Kay GN, Klein WW, Levy S, McNamara RL, Prystosky EN, Wann LS, Wyse DG. ACC/AHA/ESC guidelines for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association task Force on Practice Guidelines and the European Society of cardiology Committee for Practice Guidelines and Policy Conferences.