-
PDF
- Split View
-
Views
-
Cite
Cite
D.A. Hughes, B. Tunnage, S.T. Yeo, Drugs for exceptionally rare diseases: do they deserve special status for funding?, QJM: An International Journal of Medicine, Volume 98, Issue 11, November 2005, Pages 829–836, https://doi.org/10.1093/qjmed/hci128
Close - Share Icon Share
Abstract
Ultra-orphan drugs are medicines used to treat exceptionally rare diseases that are chronically debilitating or life-threatening. Low patient numbers make it difficult for pharmaceutical companies to recoup research and development costs, and consequently these medicines are generally expensive on a per patient basis. European Union (EU) regulations promote the development of orphan drugs; but to contain costs, some EU healthcare systems assess the cost-effectiveness of therapies when deciding if they should be funded. As ultra-orphan drugs are invariably cost-ineffective, factors in addition to cost-effectiveness need to be considered if ultra-orphan drugs are to be provided by public health services. Health service funding of ultra-orphan drugs, which varies across the EU and within the UK, has led to geographical inequities in patients' access to treatment. In some instances, support for these drugs would appear to have been approved on the basis that diseases that are rare and severe are a special case. We explore whether ultra-orphan drugs merit special status by considering efficiency, effectiveness and equity criteria. Mechanisms are discussed for creating a policy that would reduce geographical inequalities in provision across Europe.
Introduction
No single definition of orphan diseases exists: in the US the term describes conditions with a prevalence of 7 cases per 10 000 population; in Japan, 2.5 cases per 10 000 population.1 The EU criterion, applied in the UK, is a condition with a population prevalence of 5 cases or less per 10 000 population. There is no international or EU definition of ultra-orphan diseases but in the UK, the term describes conditions with a prevalence of <1 case per 50 000 population.2
EU policy supports research and development of orphan and ultra-orphan drugs.3 Incentives to pharmaceutical companies include market exclusivity for 10 years, reduction of licensing fees, assistance with marketing applications, direct access to the centralized procedure for marketing authorization, and, in some countries, provision of specific research grants. However, there is no EU policy on the use of orphan drugs by individual member states. Indeed, a survey of the first five orphan products that were granted marketing authorization by the European Medicines Agency showed a wide heterogeneity in their availability across countries. All five were available in only six countries: Austria, France, Germany, Netherlands, Portugal and the UK. In three member states (Belgium, Luxemburg and Ireland) fewer than three products were available. The timing of availability and mean price of the orphan drugs, among the member states, were also highly variable.4
Across Europe, recommendations to support the use of treatments by National Health Services vary by country. Within the UK, for instance, decisions are made regionally. In Scotland, this is the responsibility of the Scottish Medicines Consortium (SMC). In England, recommendations are made by the National Institute for Health and Clinical Excellence (NICE). In Wales, the All Wales Medicines Strategy Group (AWMSG) provides recommendations on medicines that have not been evaluated by NICE. None has specific processes for the evaluation of orphan or ultra-orphan drugs, though NICE, which has only appraised one ultra-orphan medicine (imatinib for gastrointestinal stromal tumours), is in the process of conducting a feasibility study on the relevant appraisal process.
Table 1 presents the result of a survey of the availability of the ultra-orphan drug laronidase across Europe. Laronidase is the only treatment for mucopolysaccharidosis type 1 (MPS1), a lysosomal storage disease caused by deficiency in the enzyme α-L-iduronidase. Its use is not supported in Scotland, the Netherlands, Latvia or Slovenia.
Results of a survey on the use of laronidase for MPS1 across Europe
| Country . | Availability on the National Health Service . | Appraisal status . | Comments . |
|---|---|---|---|
| Austria | Reimbursed | Not applicable | A full clinical and economic appraisal is not required for drugs that are not on the positive list (erstattungskodex). These include hospital products. |
| Belgium | Fully reimbursed, but considered on a case by case basis | Not applicable | Appraisal of the cost-effectiveness and budget impact of orphan drugs is not required. Reimbursement is at the discretion of the medical council for orphan drugs, Collège des Médecins pour les médicaments orphelins. |
| Denmark | Fully reimbursed | Not appraised | Medicines for hospital use are free for patients. |
| England | Available in some regions | Not appraised | No central guidance has been issued on the use of laronidase. However, local guidelines do not support its use in the National Health Service. |
| Finland | Fully reimbursed | Company submission included details of budget impact analysis, but no details of cost-effectiveness | Medicines for hospital use are free for patients. |
| France | Fully reimbursed | Not appraised | French hospitals receive extra funding for laronidase. Healthcare authorities usually pay for drugs without asking for details on cost-effectiveness or budget impact. However, all drugs are evaluated by the transparency commission for evidence of clinical effectiveness. |
| Germany | Fully reimbursed by the national sickness fund | Not appraised | There is no special reimbursement policy for orphan drugs. With the exception of cases where the National Health Service has restricted their use because of an appraisal by the Gemeinsame Bundesausschuss, every drug is reimbursed. |
| Greece | Partially reimbursed (75%) | Not appraised by the National Organization for Medicines | The Ministry of Social Security certified that laronidase is partially reimbursed (75%) according to an established special procedure called ‘the procedure to get unsubstituted medicines’. |
| Ireland | Fully reimbursed | ||
| Italy | Fully reimbursed by the National Health Care System for hospital use only | Approved for use by the Italian Agency for Drugs (AIFA) | The AIFA assesses potential benefits, harm and costs of drugs for use in the National Health Care System. |
| Latvia | Not reimbursed | No application made for appraisal | |
| Luxembourg | Fully reimbursed by the National Health Service for hospital use only | There is no specific policy for the reimbursement of orphan drugs. | |
| Netherlands | Not reimbursed generally, but available in specific centres | An appraisal was made of the clinical effectiveness and budget impact of laronidase | A decision, ultimately from the Dutch Minister of Health, concluded that there was insufficient clinical trial evidence on effectiveness. Assessment of cost-effectiveness became a requirement for drugs in 2005. |
| Poland | Fully reimbursed | Laronidase is provided free of charge to all Polish MPS I patients. In 2005, reimbursement is from a special program of the National Health Fund (National Health Insurance), previously it was a special program of the Ministry of Health. | |
| Portugal | Fully reimbursed | Portuguese reimbursement policy specifies that medicinal products used in certain rare diseases are fully or partially reimbursed by the government (e.g. cystic fibrosis, haemophilia). Other rare diseases have specific reimbursement policies that provide full reimbursement of all medicines (e.g. familial amyloid polyneuropathies), of a list of selected medicines (e.g. Lennox-Gastaut syndrome, Turner syndrome, etc.) or of an orphan drug when used in a rare disease (e.g. pegvisomant in acromegalic patients). | |
| Scotland | Not supported for use on the National Health Service in Scotland | Not approved on the basis that the Scottish Medicines Consortium (SMC) considered that laronidase was not a cost-effective use of healthcare resources | Evidence presented to the SMC include details of clinical and cost-effectiveness, as well as expected budgetary impact. No separate policy exists for orphan drugs. |
| Slovenia | Not reimbursed | Not appraised | There is no specific policy for the reimbursement of orphan drugs. |
| Spain | Fully reimbursed | ||
| Sweden | Fully reimbursed | Not appraised | |
| Wales | Supported for use on the National Health Service in Wales | Approved by the All Wales Medicines Strategy Group (AWMSG) | Evidence presented to the AWMSG include details of clinical and cost-effectiveness, as well as expected budgetary impact. No specific policy exists for orphan drugs. Medicines for hospital use are free for patients. |
| Country . | Availability on the National Health Service . | Appraisal status . | Comments . |
|---|---|---|---|
| Austria | Reimbursed | Not applicable | A full clinical and economic appraisal is not required for drugs that are not on the positive list (erstattungskodex). These include hospital products. |
| Belgium | Fully reimbursed, but considered on a case by case basis | Not applicable | Appraisal of the cost-effectiveness and budget impact of orphan drugs is not required. Reimbursement is at the discretion of the medical council for orphan drugs, Collège des Médecins pour les médicaments orphelins. |
| Denmark | Fully reimbursed | Not appraised | Medicines for hospital use are free for patients. |
| England | Available in some regions | Not appraised | No central guidance has been issued on the use of laronidase. However, local guidelines do not support its use in the National Health Service. |
| Finland | Fully reimbursed | Company submission included details of budget impact analysis, but no details of cost-effectiveness | Medicines for hospital use are free for patients. |
| France | Fully reimbursed | Not appraised | French hospitals receive extra funding for laronidase. Healthcare authorities usually pay for drugs without asking for details on cost-effectiveness or budget impact. However, all drugs are evaluated by the transparency commission for evidence of clinical effectiveness. |
| Germany | Fully reimbursed by the national sickness fund | Not appraised | There is no special reimbursement policy for orphan drugs. With the exception of cases where the National Health Service has restricted their use because of an appraisal by the Gemeinsame Bundesausschuss, every drug is reimbursed. |
| Greece | Partially reimbursed (75%) | Not appraised by the National Organization for Medicines | The Ministry of Social Security certified that laronidase is partially reimbursed (75%) according to an established special procedure called ‘the procedure to get unsubstituted medicines’. |
| Ireland | Fully reimbursed | ||
| Italy | Fully reimbursed by the National Health Care System for hospital use only | Approved for use by the Italian Agency for Drugs (AIFA) | The AIFA assesses potential benefits, harm and costs of drugs for use in the National Health Care System. |
| Latvia | Not reimbursed | No application made for appraisal | |
| Luxembourg | Fully reimbursed by the National Health Service for hospital use only | There is no specific policy for the reimbursement of orphan drugs. | |
| Netherlands | Not reimbursed generally, but available in specific centres | An appraisal was made of the clinical effectiveness and budget impact of laronidase | A decision, ultimately from the Dutch Minister of Health, concluded that there was insufficient clinical trial evidence on effectiveness. Assessment of cost-effectiveness became a requirement for drugs in 2005. |
| Poland | Fully reimbursed | Laronidase is provided free of charge to all Polish MPS I patients. In 2005, reimbursement is from a special program of the National Health Fund (National Health Insurance), previously it was a special program of the Ministry of Health. | |
| Portugal | Fully reimbursed | Portuguese reimbursement policy specifies that medicinal products used in certain rare diseases are fully or partially reimbursed by the government (e.g. cystic fibrosis, haemophilia). Other rare diseases have specific reimbursement policies that provide full reimbursement of all medicines (e.g. familial amyloid polyneuropathies), of a list of selected medicines (e.g. Lennox-Gastaut syndrome, Turner syndrome, etc.) or of an orphan drug when used in a rare disease (e.g. pegvisomant in acromegalic patients). | |
| Scotland | Not supported for use on the National Health Service in Scotland | Not approved on the basis that the Scottish Medicines Consortium (SMC) considered that laronidase was not a cost-effective use of healthcare resources | Evidence presented to the SMC include details of clinical and cost-effectiveness, as well as expected budgetary impact. No separate policy exists for orphan drugs. |
| Slovenia | Not reimbursed | Not appraised | There is no specific policy for the reimbursement of orphan drugs. |
| Spain | Fully reimbursed | ||
| Sweden | Fully reimbursed | Not appraised | |
| Wales | Supported for use on the National Health Service in Wales | Approved by the All Wales Medicines Strategy Group (AWMSG) | Evidence presented to the AWMSG include details of clinical and cost-effectiveness, as well as expected budgetary impact. No specific policy exists for orphan drugs. Medicines for hospital use are free for patients. |
Results of a survey on the use of laronidase for MPS1 across Europe
| Country . | Availability on the National Health Service . | Appraisal status . | Comments . |
|---|---|---|---|
| Austria | Reimbursed | Not applicable | A full clinical and economic appraisal is not required for drugs that are not on the positive list (erstattungskodex). These include hospital products. |
| Belgium | Fully reimbursed, but considered on a case by case basis | Not applicable | Appraisal of the cost-effectiveness and budget impact of orphan drugs is not required. Reimbursement is at the discretion of the medical council for orphan drugs, Collège des Médecins pour les médicaments orphelins. |
| Denmark | Fully reimbursed | Not appraised | Medicines for hospital use are free for patients. |
| England | Available in some regions | Not appraised | No central guidance has been issued on the use of laronidase. However, local guidelines do not support its use in the National Health Service. |
| Finland | Fully reimbursed | Company submission included details of budget impact analysis, but no details of cost-effectiveness | Medicines for hospital use are free for patients. |
| France | Fully reimbursed | Not appraised | French hospitals receive extra funding for laronidase. Healthcare authorities usually pay for drugs without asking for details on cost-effectiveness or budget impact. However, all drugs are evaluated by the transparency commission for evidence of clinical effectiveness. |
| Germany | Fully reimbursed by the national sickness fund | Not appraised | There is no special reimbursement policy for orphan drugs. With the exception of cases where the National Health Service has restricted their use because of an appraisal by the Gemeinsame Bundesausschuss, every drug is reimbursed. |
| Greece | Partially reimbursed (75%) | Not appraised by the National Organization for Medicines | The Ministry of Social Security certified that laronidase is partially reimbursed (75%) according to an established special procedure called ‘the procedure to get unsubstituted medicines’. |
| Ireland | Fully reimbursed | ||
| Italy | Fully reimbursed by the National Health Care System for hospital use only | Approved for use by the Italian Agency for Drugs (AIFA) | The AIFA assesses potential benefits, harm and costs of drugs for use in the National Health Care System. |
| Latvia | Not reimbursed | No application made for appraisal | |
| Luxembourg | Fully reimbursed by the National Health Service for hospital use only | There is no specific policy for the reimbursement of orphan drugs. | |
| Netherlands | Not reimbursed generally, but available in specific centres | An appraisal was made of the clinical effectiveness and budget impact of laronidase | A decision, ultimately from the Dutch Minister of Health, concluded that there was insufficient clinical trial evidence on effectiveness. Assessment of cost-effectiveness became a requirement for drugs in 2005. |
| Poland | Fully reimbursed | Laronidase is provided free of charge to all Polish MPS I patients. In 2005, reimbursement is from a special program of the National Health Fund (National Health Insurance), previously it was a special program of the Ministry of Health. | |
| Portugal | Fully reimbursed | Portuguese reimbursement policy specifies that medicinal products used in certain rare diseases are fully or partially reimbursed by the government (e.g. cystic fibrosis, haemophilia). Other rare diseases have specific reimbursement policies that provide full reimbursement of all medicines (e.g. familial amyloid polyneuropathies), of a list of selected medicines (e.g. Lennox-Gastaut syndrome, Turner syndrome, etc.) or of an orphan drug when used in a rare disease (e.g. pegvisomant in acromegalic patients). | |
| Scotland | Not supported for use on the National Health Service in Scotland | Not approved on the basis that the Scottish Medicines Consortium (SMC) considered that laronidase was not a cost-effective use of healthcare resources | Evidence presented to the SMC include details of clinical and cost-effectiveness, as well as expected budgetary impact. No separate policy exists for orphan drugs. |
| Slovenia | Not reimbursed | Not appraised | There is no specific policy for the reimbursement of orphan drugs. |
| Spain | Fully reimbursed | ||
| Sweden | Fully reimbursed | Not appraised | |
| Wales | Supported for use on the National Health Service in Wales | Approved by the All Wales Medicines Strategy Group (AWMSG) | Evidence presented to the AWMSG include details of clinical and cost-effectiveness, as well as expected budgetary impact. No specific policy exists for orphan drugs. Medicines for hospital use are free for patients. |
| Country . | Availability on the National Health Service . | Appraisal status . | Comments . |
|---|---|---|---|
| Austria | Reimbursed | Not applicable | A full clinical and economic appraisal is not required for drugs that are not on the positive list (erstattungskodex). These include hospital products. |
| Belgium | Fully reimbursed, but considered on a case by case basis | Not applicable | Appraisal of the cost-effectiveness and budget impact of orphan drugs is not required. Reimbursement is at the discretion of the medical council for orphan drugs, Collège des Médecins pour les médicaments orphelins. |
| Denmark | Fully reimbursed | Not appraised | Medicines for hospital use are free for patients. |
| England | Available in some regions | Not appraised | No central guidance has been issued on the use of laronidase. However, local guidelines do not support its use in the National Health Service. |
| Finland | Fully reimbursed | Company submission included details of budget impact analysis, but no details of cost-effectiveness | Medicines for hospital use are free for patients. |
| France | Fully reimbursed | Not appraised | French hospitals receive extra funding for laronidase. Healthcare authorities usually pay for drugs without asking for details on cost-effectiveness or budget impact. However, all drugs are evaluated by the transparency commission for evidence of clinical effectiveness. |
| Germany | Fully reimbursed by the national sickness fund | Not appraised | There is no special reimbursement policy for orphan drugs. With the exception of cases where the National Health Service has restricted their use because of an appraisal by the Gemeinsame Bundesausschuss, every drug is reimbursed. |
| Greece | Partially reimbursed (75%) | Not appraised by the National Organization for Medicines | The Ministry of Social Security certified that laronidase is partially reimbursed (75%) according to an established special procedure called ‘the procedure to get unsubstituted medicines’. |
| Ireland | Fully reimbursed | ||
| Italy | Fully reimbursed by the National Health Care System for hospital use only | Approved for use by the Italian Agency for Drugs (AIFA) | The AIFA assesses potential benefits, harm and costs of drugs for use in the National Health Care System. |
| Latvia | Not reimbursed | No application made for appraisal | |
| Luxembourg | Fully reimbursed by the National Health Service for hospital use only | There is no specific policy for the reimbursement of orphan drugs. | |
| Netherlands | Not reimbursed generally, but available in specific centres | An appraisal was made of the clinical effectiveness and budget impact of laronidase | A decision, ultimately from the Dutch Minister of Health, concluded that there was insufficient clinical trial evidence on effectiveness. Assessment of cost-effectiveness became a requirement for drugs in 2005. |
| Poland | Fully reimbursed | Laronidase is provided free of charge to all Polish MPS I patients. In 2005, reimbursement is from a special program of the National Health Fund (National Health Insurance), previously it was a special program of the Ministry of Health. | |
| Portugal | Fully reimbursed | Portuguese reimbursement policy specifies that medicinal products used in certain rare diseases are fully or partially reimbursed by the government (e.g. cystic fibrosis, haemophilia). Other rare diseases have specific reimbursement policies that provide full reimbursement of all medicines (e.g. familial amyloid polyneuropathies), of a list of selected medicines (e.g. Lennox-Gastaut syndrome, Turner syndrome, etc.) or of an orphan drug when used in a rare disease (e.g. pegvisomant in acromegalic patients). | |
| Scotland | Not supported for use on the National Health Service in Scotland | Not approved on the basis that the Scottish Medicines Consortium (SMC) considered that laronidase was not a cost-effective use of healthcare resources | Evidence presented to the SMC include details of clinical and cost-effectiveness, as well as expected budgetary impact. No separate policy exists for orphan drugs. |
| Slovenia | Not reimbursed | Not appraised | There is no specific policy for the reimbursement of orphan drugs. |
| Spain | Fully reimbursed | ||
| Sweden | Fully reimbursed | Not appraised | |
| Wales | Supported for use on the National Health Service in Wales | Approved by the All Wales Medicines Strategy Group (AWMSG) | Evidence presented to the AWMSG include details of clinical and cost-effectiveness, as well as expected budgetary impact. No specific policy exists for orphan drugs. Medicines for hospital use are free for patients. |
The SMC did not approve the use of laronidase in NHS Scotland, on the basis that it was not a cost-effective use of health care resources.5 Although laronidase is available in England, there is no central guidance, and some local guidelines do not support its use in the NHS.6 In Wales, the AWMSG approved the use of laronidase in NHS Wales, at an annual cost of £180 000 per patient. This has minimal impact on the health budget, as only two Welsh patients are currently eligible for treatment.7
In the Netherlands, the Minister of Health decides whether or not an orphan drug is to be made available, based on advice from the Netherlands Medicines Evaluation Board. In the case of laronidase, a commission of experts concluded that there was insufficient evidence to make an informed judgement on its therapeutic value. Therefore a tailor-made decision was made to limit its reimbursement to three specific academic hospitals, provided that these hospitals conducted research into the effectiveness of laronidase in MPS1. The reimbursement is limited to 2 years, to allow for collection of further evidence, after which time the Minister of Health will decide on the future policy regarding the reimbursement of laronidase.
Special status considerations
A key issue around whether public funding should support the provision of ultra-orphan drugs is whether the rarity and gravity of the condition represents a rational basis for applying a different value to health gain obtained by people with that condition. That ultra-orphan drugs are reimbursed at all, illustrates the fact that budget impact, clinical effectiveness and/or equity issues are given precedence over cost-effectiveness in decisions on resource allocation in some countries. The consequence, however, is that the opportunity cost of supporting the use of ultra-orphan drugs necessitates that patients with a more common disease, for which a cost-effective treatment is available, are denied treatment.
There are views both in favour and against granting special status for ultra-orphan drugs. These stem from arguments relating to difficulties in assessing their effectiveness and ensuring access to treatments where no other treatment exists on the one hand, and the opportunity cost of adopting treatments that are not cost-effective on the other.
Methodological issues concerning evidence on effectiveness
Decision-making bodies and reimbursement authorities require evidence on safety, efficacy, clinical and cost-effectiveness. There are a number of methodological issues, however, that make it difficult to obtain good quality comparative effectiveness data for ultra-orphan drugs. First, although randomized controlled trials are the most robust study design for hypothesis testing, it is often not possible to recruit an adequate sample size to test treatments for very rare diseases. A trial of itraconazole for the prevention of severe fungal infection in children and adults with chronic granulomatous disease, for instance, took 10 years to recruit just 39 patients.8 The FDA granted a licence for L-carnitine in genetic carnitine deficiency, based on a study of only 16 patients. Second, clinical evidence on ultra-orphan drugs for chronic diseases is often based on short-term surrogate outcomes rather than long-term effectiveness, and the relationship between the two may not be proven.
Improvements in post-marketing studies, and development of national or international registries that allow long-term follow-up on safety and effectiveness would go part way to address these issues. It is important, however, to recognize that differences in levels of evidence on clinical effectiveness for ultra-orphan drugs, vis-à-vis drugs for more prevalent conditions, are to be expected. Some authorities, such as NICE, recognize this by accepting evidence that is not necessarily based on data from randomized controlled trials.9 Less robust evidence on clinical effectiveness (and hence cost-effectiveness), therefore, may be a basis for positive discrimination favouring ultra-orphan drugs, but only when data from non-experimental sources are regarded as unacceptable by reimbursement authorities.
Limited budget impact
Given the small number of patients eligible for ultra-orphan drugs, the total cost impact on health services is limited. Even for treatments that cost £50 000 per patient per year, for instance, but for which only 50 patients in a given country are eligible, the annual net budgetary impact is likely to be no greater than £2.5m. Evidence from past decisions suggest that this level of cost is sufficiently insignificant, despite treatments not being cost-effective, to warrant funding. Furthermore, recent discussions by the Citizens Council of NICE agreed that the NHS should be prepared to pay premium prices for drugs to treat patients with very rare and severe diseases.10 However, greater improvements in health outcome of the population at large would be gained through redirecting the available resources to more cost-effective treatments.
Equity issues
The equity principle argues against special consideration for patients with rare conditions in the allocation of health care resources. The utilitarian approach to distributive justice contends that overall good (or public utility) is to be maximized. It is often expressed as ‘bringing the greatest good to the greatest number’, and normally forms the basis of economic evaluation. Investing substantial amounts of resources for rare conditions may be considered unethical from a utilitarian point of view, as it this does not maximize society's benefits. Thus, given a constrained health budget, funding ultra-orphan drugs will displace other healthcare interventions, irrespective of the net cost of the drugs. Decisions that favour orphan drugs, therefore, imply that a patient with a more common condition, and who would benefit equally, is less worthy of receiving the treatment.
A rights-based approach, in which individuals in a society are entitled to a decent minimum of health care, requires that treatment is made available for managing rare diseases. This is adopted in EU legislation, which states that patients suffering from a rare condition should be entitled to the same quality of treatment as other patients.3 In some European countries, such as Italy and the Netherlands, the right to health care is protected constitutionally.11 The French and German constitutions contain a legal obligation to assist individuals in danger. This could potentially apply to the use of treatments for life-threatening orphan diseases. However, the main problem of a rights-based approach to decisions about health care resource allocation is that even when a right to health care is embodied in national legislation, its scope is open to interpretation.12 Ultra-orphan diseases that are of genetic origin are typically chronic, debilitating and associated with reduced life-expectancy. It is unclear whether they pose sufficient imminent threat to the life of patients to constitute a right to treatment. Further, the right to a minimum standard of care would not necessarily favour rare conditions over more prevalent conditions.
The ‘rule of rescue’ proposes a commitment to non-abandonment of individuals with needs for highly specialized treatments, even in resource-constrained settings.13 While the rule attaches added weight to interventions in the face of death, it can also be a factor when life is not endangered.14 It supports the notion that society places a greater value on health gains made by individuals if there are a small number of cases, the condition is severe and no alternative treatments are available. The implementation of the rule is epitomized by cases where children with physical deformities or disfigurements are flown from poor countries to wealthier countries for treatment. Research confirms that society does place a higher value on an improvement in health when it is experienced by a person who has worse lifetime health prospects.15
Options for policy recommendations
Several possible options are available for developing policies that provide explicit criteria on whether or not funding should be available for ultra-orphan drugs.
Assigning equity weights
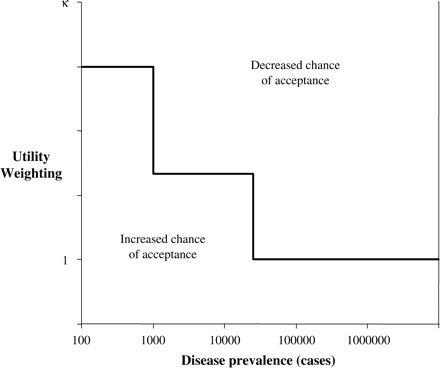
In the context of equity and prioritization of health care, Williams explored the notion that everyone should be entitled to a normal span of health.16 Equity weightings applied to QALYs would alter the distribution of health care between social classes or intergenerationally to reduce health inequalities. Similarly, an explicit QALY weighting according to disease prevalence would provide a ceiling for treatments provided for people with rare, serious conditions. This would make funding decisions transparent, and clarify the terms on which ‘priority groups’ and more powerful rivals compete for healthcare resources. Weighted QALYs increase the value of the health gain, thereby increasing the likelihood an ultra-orphan drug will have a cost-effectiveness below a given threshold. For patients with rare diseases, this reflects the limit of society's willingness to make equity-based adjustments in the distribution of health care resources (Figure 1).
Utility weighted by disease prevalence, where κ represents the utility weighting index. Different weightings are given for orphan drug status (<25 000 cases in the UK) and ultra-orphan drug status (<1000 cases).
Risk-sharing and ‘no cure, no pay’ schemes
Risk-sharing schemes are a new approach to funding expensive medications with unproven effectiveness. Chapman described a risk-sharing scheme between a pharmaceutical company and an English Health Authority.17 A guarantee on the performance targets was negotiated so that predictable health gains were achieved for a given drug expenditure. The UK Government entered similar arrangements with the manufacturers of beta interferon and glatiramer acetate,18 with agreed target treatment effects for patients with multiple sclerosis. If these are not achieved, the drug costs will be reduced to maintain cost-effectiveness at a threshold of £36 000 per QALY over 20 years. It was argued, however, that for a comparable cost, a randomized trial of beta interferon would produce a more valuable result and represent a more appropriate use of public money.19
Similar ‘no cure, no pay’ initiatives have been successfully used across Europe and the US.20 If a drug does not cure, relieve, or prevent the patient's symptoms, based on specific clinical measures or visible results, the healthcare system gets its money back. A money-back guarantee might also be applicable if the patient suffers adverse effects. However, experience is limited to treatments of common diseases (e.g. hypertension, hyperlipidaemia, erectile dysfunction and schizophrenia).
It remains to be established whether a risk-sharing or a ‘no cure, no pay’ scheme would be appropriate and workable for ultra-orphan drugs.
Clinical and pharmacogenetic criteria
Eligibility for, and hence cost related to, ultra-orphan drugs may be contained by specifying strict clinical criteria that extend beyond the licensing indications. In Ontario, Canada, for instance, reimbursement of enzyme replacement therapy for Gaucher's disease is based on actual or anticipated severity of the disease as assessed by an advisory committee of medical experts.21 Most patients with Gaucher's disease are largely asymptomatic; only those with severely disabling, if not life-threatening complications, such as severe anaemia or thrombocytopaenia, severe skeletal complications, or pulmonary hypertension are eligible for province-funded treatment. Pharmacogenetic tests that may allow potential responders to be identified, are a complimentary approach to clinical assessment.
Funding by research councils
Funding treatments for patients with rare diseases within clinical trials could be justified in the interests of scientific advancement.12 Biomedical research into treatments for rare genetic diseases could be associated with positive externalities, including a better understanding of disease pathogenesis and possibilities for new treatments for more common diseases. Public funding of such research should be from the Medical Research Council (in the UK) or other funding bodies, and not be financed by National Health Services at the expense of proven therapy.22 Specific research into health services issues may be supported by national Health Technology Assessment programmes, but there is also an onus on the pharmaceutical industry to initiate trials that address health services issues, in addition to satisfying regulatory requirements.
Dedicated funding
Clinical conditions, including cancer and diabetes, already have centralized funding to assist with service provision in meeting targets. The National Specialist Commissioning Advisory Group (NSCAG) supports specialist centres for ultra-orphan conditions at a limited number of English sites, and can provide Primary Care Organizations (PCOs) with contingency funding to support expensive treatments. Funding for some ultra-orphan drugs has recently been transferred from local budgets to central funding for a period of 2 years, relieving the pressure on those PCOs and Hospital Trusts with a cluster of patients due to hereditary characteristics.23
In France, certain high-cost drugs are made available through specific centres, which receive extra funding to support their use. In the Netherlands, expensive licensed orphan drugs may be placed on a list that allows them to be prescribed by academic hospitals. 95% of the costs of the drugs on the list are reimbursed by the Ministry of Health, with the remaining 5% being paid from the hospital budget. The total costs of the orphan drugs are not allowed to exceed 5% of total hospital drug expenditure.
Conclusions
There are arguments for, and against, ultra-orphan drugs being considered as a special case for funding by healthcare systems. Whichever argument prevails, and this is likely to be country-dependent, the focus should be on reducing geographical inequalities in patient access to treatment. In the UK, the current misalignment of national policies on funding, resulting in postcode prescribing, is unacceptable from an equity stance. A UK-wide policy on the use of these drugs in the NHS, which aims to maximize population health while aspiring to the values of the EU directive, is required. Such a policy might be a compromise between a utilitarian view and a non-abandonment approach, drawing on an open debate on whether utilities are to be weighted according to prevalence, or whether a dedicated fund should be top-sliced. Risk-sharing schemes might offset some of the high costs, while giving manufacturers incentives to produce more robust evidence on clinical effectiveness. It is clear that a complete restriction on the funding of ultra-orphan drugs is not a practical or realistic solution. Each EU member state would need to develop their own policy guidelines given that there is no prospect of a pan-European agency on drug prioritization.24
The authors wish to thank the anonymous reviewers for their comments and suggestions. Funding: research grant by Actelion Pharmaceutical UK, but the views expressed in the paper are those of the authors alone. Competing interests: Actelion is a manufacturer of orphan drugs; DAH is deputy health economist of the All Wales Medicines Strategy Group and contributed to the appraisal of laronidase.
References
Lang J, Wood SC. Development of orphan vaccines: an industry perspective.
National Institute for Clinical Excellence.
European Parliament and the Council of the European Union. Regulation (EC) No 141/2000 of the European Parliament and the Council of 16 December 1999 on orphan medicinal products.
The European Agency for the Evaluation of Medicinal Products.
Thames Valley Priorities Committees.
National Institute for Clinical Excellence.
National Institute for Clinical Excellence.
Den Exter A, Hermans H. The right to health care: a changing concept? In: Den Exter A, Hermans H, eds.
Gericke CA, Riesberg A, Busse R. Ethical issues in funding orphan drug research and development.
Hadorn DC. Setting health care priorities in Oregon. Cost-effectiveness meets the rule of rescue.
Dolan P, Shaw R, Tsuchiya A, Williams A. QALY maximisation and people's preferences: a methodological review of the literature.
Williams A. Intergenerational equity: an exploration of the ‘fair innings’ argument.
Chapman S, Reeve E, Rajaratnam G, Neary R. Setting up an outcomes guarantee for pharmaceuticals: new approach to risk sharing in primary care.
Department of Health.
Sudlow CL, Counsell CE. Problems with UK government's risk sharing scheme for assessing drugs for multiple sclerosis.
Clarke JTR, Amato D, Deber RB. Managing public payment for high-cost, high-benefit treatment: enzyme replacement therapy for Gaucher's disease in Ontario.
Department of Health.