Abstract
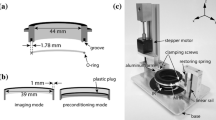
A stretch chamber has been developed in order to visualize the deformation of cells subjected to controlled uniaxial stretch of their substrate. A rectangular, custom-made, transparent silicone channel is used as a deformable substrate. Bovine aortic endothelial cells are plated at the bottom of the channel whose lateral deformation is controlled by two piezoelectric translators. The system is mounted on the stage of a confocal microscope where three-dimensional (3D) images of the cells can be acquired simultaneously in the three RGB channels. The first channel provides images of 216 nm fluorescent beads embedded in the cytoskeleton (used as internal markers). The second is used to image the shape of the nucleus revealed by live cell nucleic acid staining. The third one provides a transmitted light image of the cell outline. 3D images of the cell are taken before deformation, after uniaxial deformation of the substrate (up to 25%) and after relaxation. Results indicate that: (a) the cell closely follows the deformation imposed by the substrate with no measurable residual strain after relaxation, and (b) there is a clear mechanical coupling between the extracellular matrix and the nucleus, which deforms significantly under the applied substrate stretch. Suggesting that the nucleus can directly sense the mechanical environment of the cell, the latter result has potentially important implications for signal transduction.
Similar content being viewed by others
REFERENCES
Ashkin, A., K. Schütze, M. Dziedzic, U. Euteneuer, and M. Schliwa. Force generation of organelle transport measured in vivoby infrared laser trap. Nature (London)348:346-348, 1990.
Barbee, K. A., J. E. Macarak, and L. E. Thibault. Strain measurements in cultured vascular smooth muscle cells subjected to mechanical deformation. Ann. Biomed. Eng.22:14- 22, 1994.
Davies, P. F., and S. C. Tripathi. Mechanical stress mechanisms of the cell, an endothelial paradigm. Circ. Res.72:239-245, 1993.
Dewey, C. F., S. R. Bussolari, M. A. Gimbrone, and P. F. Davies. The dynamic response of vascular endothelial cells to fluid shear stress. J. Biomech. Eng.103:177-185, 1981.
Evans, E., K. Ritchie, and R. Merkel. Sensitive force technique to probe molecular adhesion and structural linkages at biological interfaces. Biophys. J.68:2580-2587, 1995.
Fey, E. G., K. M. Wan, and S. Penman. Epithelial cytoskeletal framework and nuclear matrix-intermediate filament scaffold: Three-dimensional organization and protein composition. J. Cell Biol.98:1973-1984, 1984.
Flaherty, J. T., J. E. Pierce, V. J. Ferrans, D. J. Patel, W. K. Tucker, and D. L. Fry. Endothelial nuclear patterns in canine arterial tree with particular reference to hemodynamic events. Circ. Res.30:23-33, 1972.
Fry, D. L. Acute vascular endothelial changes associated with increased blood velocity gradients. Circ. Res.22:165-197, 1968.
Ives, C. L., S. G. Eskin, and L. V. McIntire. Mechanical effects on endothelial cell morphology: In vitroassessment. In Vivo Cell Dev. Biol.22:500-507, 1986.
McIntosh, F. C., J. Käs, and P. A. Janmey. Elasticity of semiflexible biopolymer networks. Phys. Rev. Lett.75:4425- 4428, 1995.
Moore, J. E., E. Bürki, A. Suciu, S. Zhao, M. Burnier, H. R. Brunner, and J. J. Meister. A device for subjecting vascular endothelial cells to both fluid shear stress and circumferential cyclic stretch. Ann. Biomed. Eng.22:416-422, 1994.
Pavalko, F. M., and C. A. Otey. Role of adhesion molecule cytoplasmic domains in mediating interactions with the cytoskeleton. Proc. Soc. Exp. Biol. Med.205:282-293, 1994.
Pienta, K. J., and D. S. Coffey. Nuclear-cytoskeletal interactions: Evidence for physical connections between the nucleus and cell periphery and their alteration by transformation. J. Cell. Biochem.49:357-365, 1992.
Satcher, R. L., and C. F. Dewey. Theoretical estimates of mechanical properties of the endothelial cell cytoskeleton. Biophys. J.71:109-118, 1996.
Simon, S. I., and G. W. Schmid-Schönbein. Kinematics of cytoplasmic deformation in neutrophils during active motion. J. Biomech. Eng.112:303-310, 1990.
Sims, J. R., S. Karp, and D. E. Ingber. Altering the cellular mechanical force balance results in integrated changes in cell, cytoskeletal and nuclear shape. J. Cell. Sci.103:1215-1222, 1992.
Wang, N., J. P. Butler, and D. E. Ingber. Mechanotransduction across the cell surface and through the cytoskeleton. Science260:1124-1127, 1993.
Zhao, S., A. Suciu, T. Ziegler, J. E. Moore, J. J. Meister, E. Burki, and H. R. Brunner. Synergistic effects of fluid shear stress and cyclic circumferential stretch on vascular endothelial cell morphology and cytoskeleton. Arterioscler. Thromb. Vasc. Biol.15:1781-1786, 1995.
Zieman, F., J. Rädler, and E. Sackman. Local measurements of viscoelastic moduli of entangled actin networks using an oscillating magnetic bead micro-rheometer. Biophys. J.66:2210-2216, 1994.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Caille, N., Tardy, Y. & Meister, J.J. Assessment of Strain Field in Endothelial Cells Subjected to Uniaxial Deformation of Their Substrate. Annals of Biomedical Engineering 26, 409–416 (1998). https://doi.org/10.1114/1.132
Issue Date:
DOI: https://doi.org/10.1114/1.132