Abstract
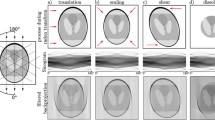
Quantification of heart valve leaflet deformation during the cardiac cycle is essential in understanding normal and pathological valvular function, as well as in the design of replacement heart valves. Due to the technical complexities involved, little work to date has been performed on dynamic valve leaflet motion. We have developed a novel experimental method utilizing a noncontacting structured laser-light projection technique to investigate dynamic leaflet motion. Using a simulated circulatory loop, a matrix of 150–200 laser light points were projected over the entire leaflet surface. To obtain unobstructed views of the leaflet surface, a stereo system of high-resolution boroscopes was used to track the light points at discrete temporal points during the cardiac cycle. The leaflet surface at each temporal point was reconstructed in three dimensions, and fit using our biquintic hermite finite element approach (Smith et al., Ann. Biomed. Eng. 26:598–611, 2001). To demonstrate our approach, we utilized a bovine pericardial bioprosthetic heart valve, which revealed regions of complex flexural deformation and substantially different shapes during the opening and closing phases. In conclusion, the current method has high spatial and temporal resolution and can reconstruct the entire surface of the cusp simultaneously. Because it is completely noncontacting, this approach is applicable to studies of fatigue and bioreactor technology for tissue engineered heart valves. © 2001 Biomedical Engineering Society.
PAC01: 8719Hh, 8780-y, 4262Be, 8719St
Similar content being viewed by others
Reference
Bellhouse, B., and F. Bellhouse. Fluid mechanics of model normal and stenosed aortic valves. Circ. Res.25:693–704, 1969.
Bellhouse, B. J., and K. G. Reid. Fluid mechanics of the aortic valve. Br. Heart J.31:391, 1969.
Black, M. M., I. C. Howard, X. C. Huang, and E. A. Patterson. A three-dimensional analysis of a bioprosthetic heart valve. J. Biomech.24:793–801, 1991.
Broom, N.Fatigue-induced damage in glutaraldehyde preserved heart valve tissue. Cardiovasc. Surg.76:202–211, 1978.
Chandran, K. B., S. H. Kim, and G. Han. Stress distribution on the cusps of a polyurethane trileaflet heart valve prosthesis in the closed position. J. Biomech.24:385–395, 1991.
Donn, A., G. Bernacca, T. Mackay, M. Gulbransen, and D. Wheatley. Laser profiling of bovine pericardial heart valves. Artif. Organs20:436–439, 1997.
Fenner, J., T. Mackay, W. Martin, and D. Wheatley. Laser profiling: A technique for the study of prosthetic heart valve leaflet motion. Physiol. Meas.16:181–193, 1995.
Ferrans, V. J., S. L. Hilbert, S. Fujita, M. Jones, and W. C. Roberts. Morphologic abnormalities in explanted bioprosthetic heart valves. In: Cardiovascular Pathology, edited by R. Virmani, J. Atkinson, and J. Fenoglio. Philadelphia, PA: W. B. Saunders, 1991, pp. 373–398.
Gabbay, S., P. Kadam, S. Factor, and T. K. Cheung. Do heart valves bioprostheses degenerate for metabolic or mechanical reasons?J. Thorac. Cardiovasc. Surg.55:208–215, 1988.
Gao, Z. B., S. Pandya, N. Hosein, M. S. Sacks, and N. H. Hwang. Bioprosthetic heart valve leaflet motion monitored by dual camera stereo photogrammetry. J. Biomech.33:199–207, 2000.
Gilliani, N. V.. Time-dependent laminar incompressible flow through a spherical cavity. J. Fluid Mech.78:99–127, 1976.
Gloeckner, D. C., K. L. Billiar, and M. S. Sacks. Effects of mechanical fatigue on the bending properties of the porcine bioprosthetic heart valve. ASAIO J.45:59–63, 1999.
Hamid, M. S., H. N. Sabbah, and P. D. Stein. Influence of stent height upon stresses on the cusps of closed bioprosthetic valves. J. Biomech.19:759–769, 1986.
Hung, T. K., and G. B. Schuessler. An analysis of the hemodynamics of the opening of aortic valves. J. Biomech.10:597–606, 1977.
Ishihara, T., V. J. Ferrans, S. W. Boyce, M. Jones, and W. C. Roberts. Structure and classification of cuspal tears and perforations in porcine bioprosthetic cardiac valves implanted in patients. Am. J. Cardiol.48:665–678, 1981.
Iyengar, A. K. S., E. G. Cape, and W. H. Neches. Dicrete subaortic stenosis: An in vitro study of the interaction between hemodynamics and aortic valve mechanics. J. Am. Soc. Echocardiography12:368, 1999.
Katz, N. M., M. J. Buckley, and R. R. Liberthson. Discrete membranous subaortic stenosis. Report of 31 patients, review of the literature, and delineation of management. Circulation56:1034–1038, 1977.
Krucinski, S., I. Vesely, M. A. Dokainish, and G. Campbell. Numerical simulation of leaflet flexure in bioprosthetic valves mounted on rigid and expansile stents. J. Biomech.26:929–943, 1993.
Lo, D., and I. Vesely. Biaxial strain analysis of the porcine aortic valve. Ann. Thorac. Surg.60:S374–S378, 1995.
Makihijani, V. B., Y. H. Dionne, P. J. Thubrikar. Three-dimensional coupled fluid-structure simulation of pericardial bioprosthetic aortic valve function. ASAIO J.43:387–392, 1997.
Marzan, G. T., K. H. A computer program for direct linear transformation solution of the collinearity condition and some applications of it. Proceedings of the Symposium on Close-Range Photogrammetric Systems, 1975, pp. 420–476.
Mercer, J. L.The movements of the dog's aortic valve studied by high speed cineangiography. Br. J. Radiol.46:344–349, 1973.
Newfeld, E. A., A. J. Muster, M. H. Paul, F. S. Idriss, and W. L. Riker. Discrete subvalvular aortic stenosis in childhood. Study of 51 patients. Am. J. Cardiol.38:53–61, 1976.
Patterson, E. A., I. C. Howard, and M. A. Thornton. A comparative study of linear and nonlinear simulations of the leaflets in a bioprosthetic heart valve during the cardiac cycle. J. Med. Eng. Technol.20:95–108, 1996.
Peskin, C. S., and A. W. Wolfe. The aortic sinus vortex. Fed. Proc.37:2784–2792, 1978.
Rosenburg, G. P. W., D. L. Landis, and W. S. Pierce. Design and evaluation of the Pennsylvania State University mock circulatory system. ASAIO J.4:41–49, 1981.
Sacks, M. S., C. J. Chuong, G. H. Templeton, and R. Peshock. In vivo 3D reconstruction and geometric characterization of the right ventricular free wall. Ann. Biomed. Eng.21:263–275, 1993.
Sacks, M., D. Gloeckner, N. Vyavahare, and R. Levy. Loss of flexural rigidity in bioprosthetic heart valves with fatigue: New findings and the relation to collagen damage. J. Heart Valve Dis. (in press).
Schoen, F. D., and C. E. Hobson. Anatomic analysis of removed prosthetic heart valves: Causes of failure of 33 mechanical valves and 58 bioprostheses, Hum. Pathol.16:545–549, 1980.
Schoen, F., and R. Levy. Pathology of substitute heart valves. J. Card. Surg.9:222–227, 1994.
Smith, D. B., M. S. Sacks, P. M. Pattany, and R. Schroeder. Fatigue-induced changes in bioprosthetic heart valve three-dimensional geometry and the relation to tissue damage. J. Heart Valve Dis.8:25–33, 1999.
Smith, D. B., M. S. Sacks, D. A. Vorp, and M. Thornton. Surface geometric analysis of anatomic structures using biquintic finite element interpolation [In Process Citation]. Ann. Biomed. Eng.28:598–611, 2000.
Swanson, W. M., and R. E. Clark. Aortic valve leaflet motion during systole. Numerical-graphical determination. Circ. Res.32:42–48, 1973.
Thornton, M. High speed dynamic, 3D surface imaging, In: Electrical Engineering. London, Ontario: University of Western Ontario Press, 1996, p. 93.
Thubrikar, M. The Aortic Valve. Boca Raton, FL: Chemical Rubber Corp., 1990, p. 221.
Thubrikar, M., J. Deck, J. Aouad, and S. Nolan. Role of mechanical stress in calcification of aortic bioprosthetic valves. J. Thorac. Cardiovasc. Surg.86:115–125, 1983.
van Steenhoven, A. A., and M. E. H. van Dongen. Model studies of the closing behaviour of the aortic valve. J. Fluid Mech.20:21–32, 1979.
Vesely, I., D. Boughner, and T. Song. Tissue buckling as a mechanism of bioprosthetic valve failure. Ann. Thorac. Surg.46:302–308, 1988.
Wheatly, D. H., J. Fisher, I. J. Reece, T. Spyt, and P. Breeze. Primary tissue failure in pericardial heart valves. J. Thorac. Cardiovasc. Surg.94:367–374, 1987.
Wipperman, F.On the fluid dynamics of the aortic valve. J. Fluid Mech.159:487–501, 1985.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Iyengar, A.K.S., Sugimoto, H., Smith, D.B. et al. Dynamic In Vitro Quantification of Bioprosthetic Heart Valve Leaflet Motion Using Structured Light Projection. Annals of Biomedical Engineering 29, 963–973 (2001). https://doi.org/10.1114/1.1415523
Issue Date:
DOI: https://doi.org/10.1114/1.1415523