Abstract
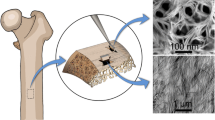
Mechanical loading has been implicated as a powerful driving mechanism for interstitial fluid flow through bone. However, little information is available with regard to the morphology of bone fluid spaces, e.g., the canalicular wall, which would be expected to dictate the type of flow regime developing in the lacunocanalicular system under mechanical loads. The purpose of this study was to examine the fine structure of the lacunocanalicular system in cortical bone using atomic force microscopy (AFM), resin casting methods, and selective etching of the specimen surface. A resincast replica of the canalicular wall was produced and surface morphology and dimensions were observed using AFM in tapping mode. Material contrast was obtained using surface potential measurements. A striped pattern perpendicular to the canaliculus long axis with a periodicity of 125 nm dominated the structure of the canalicular wall; it is likely that this was caused by the imprint of collagen fibrils arranged in parallel, lining the canaliculus wall. The largest dimension measured for canalicular diameter was on the order of 500 nm. The regular dips and ridges caused by the collagen that lines the wall are a source of roughness which may influence shear stresses imparted by the fluid on the cell surface as well as mixing of solutes within the lacunocanalicular system. In addition, the lacunocanalicular wall lining is likely to affect physicochemical interactions between the fluid and bone matrix. This has important implications for modeling and understanding the microfluid mechanics and rheology of the fluid-filled lacunocanalicular network. © 2001 Biomedical Engineering Society.
PAC01: 8719Tt, 8764Dz
Similar content being viewed by others
REFERENCES
Atkinson, P. J., and A. S. Hallsworth. The spatial structure of bone. In: Progress in Anatomy, edited by R. J. Harrision and V. Navaratman. Cambridge: Cambridge University Press, 1982, Vol. 2, pp. 179–199.
Bagi, A., M. Gandolfi, N. Roveri, and G. Valdre. In vitro calcified tendon collagen: An atomic force and scanning electron microscopy investigation. Biomaterials 18:657–665, 1997.
Bonucci, E., and G. Gherardi. Osteocyte ultrastructure in renal osteodystrophy. Virchows Arch. A Pathol. Anat. Histol. 373:213–231, 1977.
Burger, E. H., and J. Klein-Nulend. Mechanotransduction in bone-Role of the lacunocanalicular network. FASEB J. 13:S101-S112, 1999.
Castenholz, A. Interpretation of structural patterns appearing on corrosion casts of small blood and initial lymphatic vessels. Scanning Microsc. 3:315–325, 1989.
Castenholz, A. The outer surface morphology of blood vessels as revealed in scanning electron microscopy in resin cast, noncorroded tissue specimens. Scan. Electron Microsc. 4:1955–1962, 1983.
Cooper, R. R., J. W. Milgram, and R. A. Robinson. Morphology of the osteon. An electron microscopic study. J. Bone Jt. Surg. 48-A:1239–1271, 1966.
Cowin, S. C., S. Weinbaum, and Y. Zeng. A case for bone canaliculi as the anatomical site of strain generated streaming potentials. J. Biomech. 28:1281–1297, 1995.
Curtis, T. A., S. H. Ashrafi, and D. F. Weber. Canalicular communication in the cortices of human long bones. Anat. Rec. 212:336–344, 1985.
Giraud-Guille, M. M. Twisted plywood architecture of collagen fibrils in human compact bone osteons. Calcif. Tissue Int. 42:167–180, 1988.
Guzelsu, N., and W. R. Walsh. Streaming potential of intact wet bone. J. Biomech. 23:673–685, 1990.
Hein, H.-J., and L. Weisser. The presentation of hydroylapatite in bone by scanning probe microscopy. J. Biomech. 31:22, 1998.
Ismail, O. S., and D. F. Weber. Light and scanning electron microscopic observations of the canalicular system in human cellular cementum. Anat. Rec. 222:121–127, 1988.
Jacobs, H. O., H. F. Knapp, S. Müller, and A. Stemmer. Surface potential mapping: A qualitative material contrast in SPM. Ultramicroscopy 69:39–49, 1997.
Jacobs, H. O., H. F. Knapp, and A. Stemmer. Practical aspects of kelvin probe microscopy. Rev. Sci. Instrum. 70:1756–1760, 1999.
Knapp, H. F., G. C. Reilly, A. Stemmer, P. Niederer, and M. L. Knothe Tate. Development of preparation methods for and insights obtained from atomic force microscopy of fluid spaces in cortical bone. Scanning (in press).
Knothe Tate, M. L., P. Niederer, and U. Knothe. In vivo tracer transport through the lacunocanalicular system of rat bone in an environment devoid of mechanical loading. Bone 22:107–117, 1998.
Knothe Tate, M. L., A. Tami, P. Nasser, R. Steck, and M. B. Schaffler. Permeability characteristics of different molecular tracers in loaded and unloaded bone. Trans. Orthopaed. Res. Soc. 26:138, 2001.
Knothe Tate, M. L., and U. Knothe. An ex vivo model to study transport processes and fluid flow in loaded bone. J. Biomech. 33:247–254, 2000.
Knothe Tate, M. L., R. Steck, M. R. Forwood, and P. Niederer. In vivo demonstration of load-induced fluid flow in the rat tibia and its potential implications for processes associated with functional adaptation. J. Exp. Biol. 203:2737–2745, 2000.
Knothe Tate, M. L. Interstitial fluid flow. In: Bone Biomechanics Handbook, edited by S. C. Cowin. New York: CRC, 2001, pp. 22–1-22-9.
Kratky, R. G., C. M. Zeindler, D. K. C. Lo, and M. R. Roach. Quantitative measurement from vascular casts. Scanning Microsc. 3:937–943, 1989.
Lees, S., K. S. Prostak, V. K. Ingle, and K. Kjoller. The loci of mineral in turkey leg tendon as seen by atomic force microscope and electron microscopy. Calcif. Tissue Int. 55:180–189, 1994.
Luk, S. C., C. Nopajaroonsri, and G. T. Simon. The ultrastructure of cortical bone in young adult rabbits. J. Ultrastruct. Res. 46:184–205, 1974.
Marti, O., H. F. Knapp, M. Radmacher, M. Fritz, J. P. Cleveland, P. K. Hansma, and J. Colchero. Module 1.3.2, AFM signals and imaging modes. In: Procedures in Scanning Probe Microscopies, edited by R. J. Colton, A. Engel, J. E. Frommer, H. E. Gaub, A. A. Gewirth, R. Guckenberger, J. Rabe, W. M. Heckl, and B. Parkinson. Chichester: Wiley, 1998, pp. 105–121.
Martin, D. M., A. S. Hallsworth, and T. Buckley. A method for the study of internal spaces in hard tissue matrices by SEM, with special reference to dentine. J. Microsc. 112:345–352, 1978.
Nicolella, D. P., D. E. Moravits, A. J. Siller-Jackson, R. J. Railsback, S. F. Timmons, K. J. Jepsen, D. T. Davy, and J. Lankford. Ultrastructural characterization of damaged cortical bone using atomic force microscopy. In: Proceedings of the Bioengineering Conference BED-Vol. 42, edited by V. K. Goel, R. L. Spiker, G. A. Ateshian, and L. J. Soslowsky. New York: ASME, 1999, pp. 319–320.
Ráliš, Z. A., and I. G. Turner. Two phases of the bone mineral as revealed by the high-resolution scanning electron microscope on ion-etched bone surfaces and as seen on surfaces untreated and chemically etched. Microsc. Acta 84:385–400, 1981.
Raspanti, M., A. Alessandrini, V. Ottani, and A. Ruggeri. Direct visualization of collagen-bound proteoglycans by tappingmode atomic force microscopy. J. Struct. Biol. 119:118–122, 1997.
Sauren, Y. M. H. F., R. H. P. Mieremet, C. G. Groot, and J. P. Scherft. An electron microscope study of the presence of proteoglycans in the mineralized matrix of rat and human compact lamellar bone. Anat. Rec. 232:36–44, 1992.
Scherft, J. P. The lamina limitans of the organic matrix of calcified cartilage and bone. J. Ultrastruct. Res. 38:318–331, 1972.
Sims, P. A., and R. M. Albrecht. Corrosion casting in the reproduction of the microsurface topography of fibrillar collagen. Microsc. Microanal. 5:99–105, 1999.
Skerry, T. M., R. Suswillo, A. J. El Haj, N. N. Ali, R. A. Dodds, and L. E. Lanyon. Load-induced proteoglycan orientation in bone tissue in vivo and in vitro. Calcif. Tissue Int. 46:318–326, 1990.
Takagi, M., M. Maeno, A. Kagami, Y. Takahashi, and K. Otsuka. Biochemical and immunocytochemical characterization of mineral binding proteoglycans in rat bone. J. Histochem. Cytochem. 39:41–50, 1991.
Tao, N. J., S. M. Lindsay, and S. Lees. Measuring the microelastic properties of biological material. Biophys. J. 63:1165–1169, 1992.
Weinbaum, S., S. C. Cowin, and Y. Zeng. A model for the excitation of osteocytes by mechanical loading-induced bone fluid shear stresses. J. Biomech. 27:339–360, 1994.
Wiesmann, H.-P., L. Chi, U. Stratmann, U. Plate, H. Fuchs, U. Joos, and H. J. Höhling. Sutural mineralization of rat calvaria characterized by atomic force microscopy and transmission electron microscopy. Cell Tissue Res. 294:93–97, 1998.
You, J., C. E. Yellowley, H. J. Donahue, Y. Zhang, Q. Chen, and C. R. Jacobs. Substrate deformation levels associated with routine physical activity are less stimulatory to bone cells relative to loading-induced oscillatory fluid flow. J. Biomed. Eng. 122:387–393, 2000.
You L., S. C. Cowin, and S. Weinbaum. Strain amplification in the bone mechanosensory system. In: Advances in Bioengineering BED-Vol. 43, edited by J. S. Wayne. New York: ASME, 1999, pp. 171–172.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Reilly, G.C., Knapp, H.F., Stemmer, A. et al. Investigation of the Morphology of the Lacunocanalicular System of Cortical Bone Using Atomic Force Microscopy. Annals of Biomedical Engineering 29, 1074–1081 (2001). https://doi.org/10.1114/1.1424910
Issue Date:
DOI: https://doi.org/10.1114/1.1424910