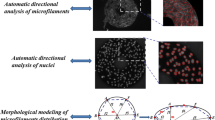
Abstract
Endothelial cells elongate and align with the direction of applied fluid shear stress. Previously, automated methods for analysis of cell orientation distribution have used Fourier- or fractal-based methods. We used intensity gradients in images of control and sheared endothelial cells to measure orientation distributions. Automated measurements of mean orientation and angular deviation compared favorably with manual measurements. There was a significantly greater angular deviation in images of control cells compared with sheared cells. Automated methods were also used to quantify organization of cytoskeletal fibers using the local angular deviation and a measure of the local coalignment of fibers called the coalignment ratio. The local angular deviation of microtubules and microfilaments was significantly smaller in sheared cells compared with control. The coalignment of cytoskeletal fibers was significantly greater in sheared cells. We conclude that image intensity gradients can be used rapidly, accurately, and objectively to measure cell orientation distributions and cytoskeletal filament organization. © 1999 Biomedical Engineering Society.
PAC99: 8716Ka, 8717-d, 8719Rr, 8764Rr, 0705Pj
Similar content being viewed by others
REFERENCES
Chaudhuri, B. B., P. Kundu, and N. Sarkar. Detection and gradation of oriented texture. Pattern Recogn. Lett. 14:147–153, 1993.
Dartsch, P. C., and E. Betz. Response of cultured endothelial cells to mechanical stimulation. Basic Res. Cardiol. 84:268–281, 1989.
Dewey, Jr., C. F., S. R. Bussolari, M. A. Gimbrone, Jr., and P. F. Davies. The dynamic response of vascular endothelial cells to fluid shear stress. J. Biomech. Eng. 103:177–185, 1981.
Eskin, S. G., C. L. Ives, L. V. McIntire, and L. T. Navarro. Response of cultured endothelial cells to steady flow. Microvasc. Res. 28:87–94, 1984.
Fisher, N. I. Statistical Analysis of Circular Data. Cambridge: Cambridge University Press, 1993.
Frangos, J., S. Eskin, L. McIntire, and C. Ives. Flow effects on prostacyclin production by cultured human endothelial cells. Science 225:1477–1479, 1985.
Galbraith, C. G., R. Skalak, and S. Chien. Shear stress induces spatial reorganization of the endothelial cell cytoskeleton. Cell Motil. Cytoskeleton 40:317–330, 1998.
Karlon, W. J., J. W. Covell, A. D. McCulloch, and J. H. Omens. Automated measurement of myofiber disarray in transgenic mice with ventricular expression of ras. Anat. Rec. 252:612–625, 1998.
Kohler, M., M. Aufderheide, and D. Ramm. Method for the description of differences in the filamentous structure of the cytoskeleton in cultured cells. Toxicol. Lett. 72:33–42, 1994.
Lee, T.-Y. J., A. Rosenthal, and A. I. Gotlieb. Transition of aortic endothelial cells from resting to migrating cells is associated with three sequential patterns of microfilament organization. J. Vasc. Res. 33:13–24, 1996.
Levesque, M. J., and R. M. Nerem. The elongation and orientation of cultured endothelial cells in response to shear stress. J. Biomech. Eng. 107:341–347, 1985.
Malek, A. M., and S. Izumo. Mechanism of endothelial cell shape change and cytoskeletal remodeling in response to fluid shear stress. J. Cell. Sci. 109(4):713–726, 1996.
Palmer, B., and R. Bizios. Quantitative characterization of vascular endothelial cell morphology and orientation using Fourier transform analysis. J. Biomech. Eng. 119:159–165, 1997.
Petroll, W. M., H. D. Cavanagh, P. Barry, P. Andrews, and J. V. Jester. Quantitative analysis of stress fiber orientation during corneal wound contraction. J. Cell. Sci. 104:353–363, 1993.
Remuzzi, A., C. F. Dewey, Jr., P. F. Davies, and M. A. Gimbrone, Jr., Orientation of endothelial cells in shear fields in vitro. Biorheology 21:617–630, 1984.
Sato, M., and N. Ohshima. Flow-induced changes in shape and cytoskeletal structure of vascular endothelial cells. Biorheology 31:143–153, 1994.
Sterpetti, A. V., A. Cucina, L. Santoro D'Angelo, B. Cardillo, and A. Cavallaro. Response of arterial smooth muscle cells to laminar flow. J. Cardiovasc. Surg. 33:619–624, 1992.
Thomason, D. B., O. Anderson, II., and V. Menon. Fractal analysis of cytoskeleton rearrangement in cardiac muscle during head-down tilt. J. Appl. Physiol. 81:1522–1527, 1996.
Thoumine, O., T. Ziegler, P. R. Girard, and R. M. Nerem. Elongation of confluent endothelial cells in culture: The importance of fields of force in the associated alterations of their cytoskeletal structure. Exp. Cell Res. 219:427–441, 1995.
Vassy, J., M. Beil, T. Irinopoulou, and J. P. Rigaut. Quantitative image analysis of cytokeratin filament distribution during fetal rat liver development. Hepatology 23:630–638, 1996.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Karlon, W.J., Hsu, PP., Li, S. et al. Measurement of Orientation and Distribution of Cellular Alignment and Cytoskeletal Organization. Annals of Biomedical Engineering 27, 712–720 (1999). https://doi.org/10.1114/1.226
Issue Date:
DOI: https://doi.org/10.1114/1.226